Immunoassay with Novel Paired Antibodies for Detection of Lipoarabinomannan in the Pleural Fluid and Plasma of Patients with Tuberculous Pleurisy
- PMID: 37764103
- PMCID: PMC10535579
- DOI: 10.3390/microorganisms11092259
Immunoassay with Novel Paired Antibodies for Detection of Lipoarabinomannan in the Pleural Fluid and Plasma of Patients with Tuberculous Pleurisy
Abstract
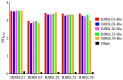
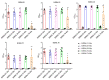
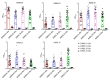
Tuberculous pleurisy (TP) is one of the most common forms of extrapulmonary tuberculosis, but its diagnosis is challenging. Lipoarabinomannan (LAM) antigen is a biomarker for Mycobacterium tuberculosis (Mtb) infection. LAM detection has potential as an auxiliary diagnostic method for TP. We have successfully generated five rabbit anti-LAM monoclonal antibodies (BJRbL01, BJRbL03, BJRbL20, BJRbL52, and BJRbL76). Here, anti-LAM antibodies were tested to detect LAM in the pleural fluid and plasma of patients with TP by sandwich enzyme-linked immunosorbent assays (ELISAs). The results revealed that all of the anti-LAM antibodies were successfully used as capture and detection antibodies in sandwich ELISAs. The BJRbL01/BJRbL01-Bio pair showed better performance than the other antibody pairs for detecting mycobacterial clinical isolates and had a limit of detection of 62.5 pg/mL for purified LAM. LAM levels were significantly higher in the pleural fluid and plasma of patients with TP than in those of patients with malignant pleural effusion or the plasma of non-TB, and LAM levels in the pleural fluid and plasma were positively correlated. Moreover, LAM levels in the pleural fluid sample were significantly higher in confirmed TP patients than in clinically diagnosed TP patients. Our studies provide novel LAM detection choices in the pleural fluid and plasma of TP patients and indicate that LAM detection assay has an auxiliary diagnostic value for TP, which may help to improve the diagnosis of TP.
Keywords: lipoarabinomannan; plasma; pleural fluid; sandwich ELISA; tuberculous pleurisy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Developing a method to detect lipoarabinomannan in pleural fluid and assessing its diagnostic efficacy for tuberculous pleural effusion.Heliyon. 2023 Aug 6;9(8):e18949. doi: 10.1016/j.heliyon.2023.e18949. eCollection 2023 Aug. Heliyon. 2023. PMID: 37600371 Free PMC article.
-
Generation of mycobacterial lipoarabinomannan-specific monoclonal antibodies and their ability to identify mycobacterium isolates.J Microbiol Immunol Infect. 2021 Jun;54(3):437-446. doi: 10.1016/j.jmii.2020.02.005. Epub 2020 Feb 20. J Microbiol Immunol Infect. 2021. PMID: 32146163
-
Lipoarabinomannan in sputum to detect bacterial load and treatment response in patients with pulmonary tuberculosis: Analytic validation and evaluation in two cohorts.PLoS Med. 2019 Apr 12;16(4):e1002780. doi: 10.1371/journal.pmed.1002780. eCollection 2019 Apr. PLoS Med. 2019. PMID: 30978194 Free PMC article.
-
Is Xpert MTB/RIF appropriate for diagnosing tuberculous pleurisy with pleural fluid samples? A systematic review.BMC Infect Dis. 2018 Jun 25;18(1):284. doi: 10.1186/s12879-018-3196-4. BMC Infect Dis. 2018. PMID: 29940951 Free PMC article.
-
The value of single or combined use of pleural fluid interferon gamma release assay in the diagnosis of tuberculous pleurisy.Trop Med Int Health. 2021 Nov;26(11):1356-1366. doi: 10.1111/tmi.13659. Epub 2021 Aug 1. Trop Med Int Health. 2021. PMID: 34297877
Cited by
-
Development and assessment of a novel magnetic nanoparticle antibody-conjugate and aptamer-based assay (MNp-Ab-Ap assay) for the rapid diagnosis of pleural tuberculosis.Nanotheranostics. 2025 Jan 1;9(1):20-30. doi: 10.7150/ntno.95332. eCollection 2025. Nanotheranostics. 2025. PMID: 39744100 Free PMC article.
References
-
- WHO . Global Tuberculosis Report 2022. WHO; Geneva, Switzerland: 2022.
-
- Kang W., Liu S., Du J., Tang P., Chen H., Liu J., Ma J., Li M., Qin J., Shu W., et al. Epidemiology of concurrent extrapulmonary tuberculosis in inpatients with extrapulmonary tuberculosis lesions in China: A large-scale observational multi-centre investigation. Int. J. Infect. Dis. 2022;115:79–85. doi: 10.1016/j.ijid.2021.11.019. - DOI - PubMed
LinkOut - more resources
Full Text Sources

