Host-Derived Extracellular Vesicles in Blood and Tissue Human Protozoan Infections
- PMID: 37764162
- PMCID: PMC10536481
- DOI: 10.3390/microorganisms11092318
Host-Derived Extracellular Vesicles in Blood and Tissue Human Protozoan Infections
Abstract
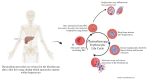
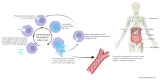
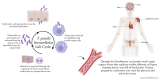
Blood and tissue protozoan infections are responsible for an enormous burden in tropical and subtropical regions, even though they can also affect people living in high-income countries, mainly as a consequence of migration and travel. These pathologies are responsible for heavy socio-economic issues in endemic countries, where the lack of proper therapeutic interventions and effective vaccine strategies is still hampering their control. Moreover, the pathophysiological mechanisms associated with the establishment, progression and outcome of these infectious diseases are yet to be fully described. Among all the players, extracellular vesicles (EVs) have raised significant interest during the last decades due to their capacity to modulate inter-parasite and host-parasite interactions. In the present manuscript, we will review the state of the art of circulating host-derived EVs in clinical samples or in experimental models of human blood and tissue protozoan diseases (i.e., malaria, leishmaniasis, Chagas disease, human African trypanosomiasis and toxoplasmosis) to gain novel insights into the mechanisms of pathology underlying these conditions and to identify novel potential diagnostic markers.
Keywords: Chagas’ disease; biomarkers; extracellular vesicles; host–pathogen interaction; human African trypanosomiasis; human leishmaniasis; malaria; protozoan infections; toxoplasmosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

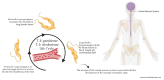
Similar articles
-
The roles of parasite-derived extracellular vesicles in disease and host-parasite communication.Parasitol Int. 2021 Aug;83:102373. doi: 10.1016/j.parint.2021.102373. Epub 2021 Apr 29. Parasitol Int. 2021. PMID: 33933651 Review.
-
Cross-reactivity between antibodies in the sera of individuals with leishmaniasis, toxoplasmosis, and Chagas' disease and antigens of the blood-stage forms of Plasmodium falciparum determined by indirect immunofluorescence.Am J Trop Med Hyg. 1995 Aug;53(2):202-5. doi: 10.4269/ajtmh.1995.53.202. Am J Trop Med Hyg. 1995. PMID: 7677225
-
Potential of extracellular vesicles in the pathogenesis, diagnosis and therapy for parasitic diseases.J Extracell Vesicles. 2024 Aug;13(8):e12496. doi: 10.1002/jev2.12496. J Extracell Vesicles. 2024. PMID: 39113589 Free PMC article. Review.
-
Extracellular Vesicles: Potential Role in Remote Signaling and Inflammation in Trypanosoma cruzi-Triggered Disease.Front Cell Dev Biol. 2021 Dec 20;9:798054. doi: 10.3389/fcell.2021.798054. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34988085 Free PMC article. Review.
-
European strategies against the parasite transfusion risk.Transfus Clin Biol. 2005 Feb;12(1):1-4. doi: 10.1016/j.tracli.2004.12.001. Transfus Clin Biol. 2005. PMID: 15814284
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

