Response Surface Methodology Application for Bacteriophage-Antibiotic Antibiofilm Activity Optimization
- PMID: 37764196
- PMCID: PMC10536537
- DOI: 10.3390/microorganisms11092352
Response Surface Methodology Application for Bacteriophage-Antibiotic Antibiofilm Activity Optimization
Abstract
Phage-antibiotic combination-based protocols are presently under heightened investigation. This paradigm extends to engagements with bacterial biofilms, necessitating novel computational approaches to comprehensively characterize and optimize the outcomes achievable via these combinations. This study aimed to explore the Response Surface Methodology (RSM) in optimizing the antibiofilm activity of bacteriophage-antibiotic combinations. We employ a combination of antibiotics (gentamicin, meropenem, amikacin, ceftazidime, fosfomycin, imipenem, and colistin) alongside the bacteriophage vB_AbaP_AGC01 to combat Acinetobacter baumannii biofilm. Based on the conducted biofilm challenge assays analyzed using the RSM, the optimal points of antibiofilm activity efficacy were effectively selected by applying this methodology, enabling the quantifiable mathematical representations. Subsequent optimization showed the synergistic potential of the anti-biofilm that arises when antibiotics are judiciously combined with the AGC01 bacteriophage, reducing biofilm biomass by up to 80% depending on the antibiotic used. The data suggest that the phage-imipenem combination demonstrates the highest efficacy, with an 88.74% reduction. Notably, the lower concentrations characterized by a high maximum reduction in biofilm biomass were observed in the phage-amikacin combination at cA = 0.00195 and cP = 0.38 as the option that required minimum resources. It is worth noting that only gentamicin antagonism between the phage and the antibiotic was detected.
Keywords: antimicrobials; biofilm; mathematical modeling; phages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




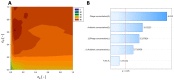
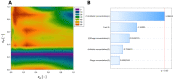
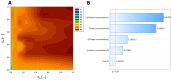
References
-
- Grygorcewicz B., Wojciuk B., Roszak M., Łubowska N., Błażejczak P., Jursa-Kulesza J., Rakoczy R., Masiuk H., Dołęgowska B. Environmental Phage-Based Cocktail and Antibiotic Combination Effects on Acinetobacter baumannii Biofilm in a Human Urine Model. Microb. Drug Resist. 2021;27:25–35. doi: 10.1089/mdr.2020.0083. - DOI - PubMed
-
- Schooley R.T., Biswas B., Gill J.J., Hernandez-Morales A., Lancaster J., Lessor L., Barr J.J., Reed S.L., Rohwer F., Benler S., et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017;61:e00954-17. doi: 10.1128/AAC.00954-17. - DOI - PMC - PubMed
-
- Galtier M., De Sordi L., Sivignon A., De Vallée A., Maura D., Neut C., Rahmouni O., Wannerberger K., Darfeuille-Michaud A., Desreumaux P., et al. Bacteriophages targeting adherent invasive Escherichia coli strains as a promising new treatment for Crohn’s disease. J. Crohn’s Colitis. 2017;11:840–847. doi: 10.1093/ecco-jcc/jjw224. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

