The Role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis
- PMID: 37764202
- PMCID: PMC10537357
- DOI: 10.3390/microorganisms11092358
The Role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis
Abstract
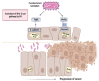
In recent years, several studies have suggested a strong association of microorganisms with several human cancers. Two periodontopathogenic species in particular have been mentioned frequently: Fusobacterium nucleatum (F. nucleatum) and Porphyromonas gingivalis. Chronic periodontal disease has been reported to be a risk factor for oral squamous cell carcinoma (OSCC), colorectal cancer (CRC) and pancreatic cancer. F. nucleatum is a Gram-negative anaerobic bacterium that lives in the oral cavity, urogenital, intestinal and upper digestive tract. It plays a significant role as a co-aggregation factor, with almost all bacterial species that participate in oral plaque formation acting as a bridge between early and late colonizers. F. nucleatum, gives an important inflammatory contribution to tumorigenesis progression and is associated with epithelial-derived malignancies, such as OSCC and CRC. F. nucleatum produces an adhesion protein, FadA, which binds to VE-cadherin on endothelial cells and to E-cadherins on epithelial cells. The last binding activates oncogenic pathways, such as Wnt/βcatenin, in oral and colorectal carcinogenesis. F. nucleatum also affects immune response because its Fap2 protein interacts with an immune receptor named TIGIT present on some T cells and natural killer cells inhibiting immune cells activities. Morover, F. nucleatum release outer membrane vesicles (OMVs), which induce the production of proinflammatory cytokines and initiating inflammation. F. nucleatum migrates from the oral cavity and reaches the colon hematogenously but it is not known if in the bloodstream it reaches the CRC as free, erythrocyte-bound bacteria or in OMV. F. nucleatum abundance in CRC tissue has been inversely correlated with overall survival (OS). The prevention and treatment of periodontal disease through the improvement of oral hygiene should be included in cancer prevention protocols. FadA virulence factors may also serve as novel targets for therapeutic intervention of oral and colorectal cancer.
Keywords: Wnt/βcatenin; colorectal cancer; immune response; oral microbiota; oral squamous cell carcinoma; periodontal bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dejea C.M., Fathi P., Craig J.M., Boleij A., Taddese R., Geis A.L., Wu X., DeStefano Shields C.E., Hechenbleikner E.M., Huso D.L., et al. Patients with Familial Adenomatous Polyposis Harbor Colonic Biofilms Containing Tumorigenic Bacteria. Science. 2018;359:592–597. doi: 10.1126/science.aah3648. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

