Therapeutic Antibodies in Medicine
- PMID: 37764213
- PMCID: PMC10535987
- DOI: 10.3390/molecules28186438
Therapeutic Antibodies in Medicine
Abstract
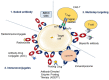
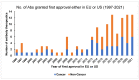
Antibody engineering has developed into a wide-reaching field, impacting a multitude of industries, most notably healthcare and diagnostics. The seminal work on developing the first monoclonal antibody four decades ago has witnessed exponential growth in the last 10-15 years, where regulators have approved monoclonal antibodies as therapeutics and for several diagnostic applications, including the remarkable attention it garnered during the pandemic. In recent years, antibodies have become the fastest-growing class of biological drugs approved for the treatment of a wide range of diseases, from cancer to autoimmune conditions. This review discusses the field of therapeutic antibodies as it stands today. It summarizes and outlines the clinical relevance and application of therapeutic antibodies in treating a landscape of diseases in different disciplines of medicine. It discusses the nomenclature, various approaches to antibody therapies, and the evolution of antibody therapeutics. It also discusses the risk profile and adverse immune reactions associated with the antibodies and sheds light on future applications and perspectives in antibody drug discovery.
Keywords: drug development; drug discovery; medicine; protein engineering; therapeutic antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

