Soybean Antigen Protein-Induced Intestinal Barrier Damage by Trigging Endoplasmic Reticulum Stress and Disordering Gut Microbiota in Weaned Piglets
- PMID: 37764275
- PMCID: PMC10534728
- DOI: 10.3390/molecules28186500
Soybean Antigen Protein-Induced Intestinal Barrier Damage by Trigging Endoplasmic Reticulum Stress and Disordering Gut Microbiota in Weaned Piglets
Abstract
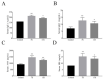
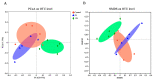
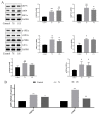
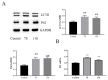
Endoplasmic reticulum (ER) stress is a crucial factor in the pathogenesis of intestinal diseases. Soybean antigenic proteins (β-conglycinin and soy glycinin) induce hypersensitivity reactions and intestinal barrier damage. However, whether this damage is associated with ER stress, autophagy, and the gut microbiome is largely unclear. Therefore, in this study, we aimed to investigate the effect of dietary supplementation with soy glycinin (11S glycinin) and β-conglycinin (7S glycinin) on intestinal ER stress, autophagy, and flora in weaned piglets. Thirty healthy 21-day-old weaned "Duroc × Long White × Yorkshire" piglets were randomly divided into three groups and fed a basic, 7S-supplemented, or 11S-supplemented diet for one week. The results indicated that 7S/11S glycinin disrupted growth performance, damaged intestinal barrier integrity, and impaired goblet cell function in piglets (p < 0.05). Moreover, 7S/11S glycinin induced ER stress and blocked autophagic flux in the jejunum (p < 0.05) and increased the relative abundance of pathogenic flora (p < 0.01) and decreased that of beneficial flora (p < 0.05). In conclusion, 7S/11S glycinin induces intestinal ER stress, autophagic flux blockage, microbiota imbalance, and intestinal barrier damage in piglets.
Keywords: autophagy; endoplasmic reticulum stress; gut microbiota; soybean glycinin; β-conglycinin.
Conflict of interest statement
All authors report no conflict of interest.
Figures







Similar articles
-
Soybean glycinin and β-conglycinin damage the intestinal barrier by triggering oxidative stress and inflammatory response in weaned piglets.Eur J Nutr. 2023 Oct;62(7):2841-2854. doi: 10.1007/s00394-023-03188-8. Epub 2023 Jun 26. Eur J Nutr. 2023. PMID: 37358571
-
Soybean Glycinin- and β-Conglycinin-Induced Intestinal Damage in Piglets via the p38/JNK/NF-κB Signaling Pathway.J Agric Food Chem. 2018 Sep 12;66(36):9534-9541. doi: 10.1021/acs.jafc.8b03641. Epub 2018 Aug 31. J Agric Food Chem. 2018. PMID: 30139257
-
4-Phenylbutyric Acid Attenuates Soybean Glycinin/β-Conglycinin-Induced IPEC-J2 Cells Apoptosis by Regulating the Mitochondria-Associated Endoplasmic Reticulum Membrane and NLRP-3.J Agric Food Chem. 2024 Mar 20;72(11):5926-5934. doi: 10.1021/acs.jafc.3c09630. Epub 2024 Mar 8. J Agric Food Chem. 2024. PMID: 38457471
-
Structural and functional analysis of various globulin proteins from soy seed.Crit Rev Food Sci Nutr. 2015;55(11):1491-502. doi: 10.1080/10408398.2012.700340. Crit Rev Food Sci Nutr. 2015. PMID: 24915310 Review.
-
Gut microbiota modulate intestinal inflammation by endoplasmic reticulum stress-autophagy-cell death signaling axis.J Anim Sci Biotechnol. 2025 May 2;16(1):63. doi: 10.1186/s40104-025-01196-8. J Anim Sci Biotechnol. 2025. PMID: 40312439 Free PMC article. Review.
Cited by
-
Simplex and multiplex CRISPR/Cas9-mediated knockout of grain protease inhibitors in model and commercial barley improves hydrolysis of barley and soy storage proteins.Plant Biotechnol J. 2025 Jun;23(6):2418-2428. doi: 10.1111/pbi.70065. Epub 2025 Mar 27. Plant Biotechnol J. 2025. PMID: 40147495 Free PMC article.
-
The role of lycopene in alleviating soybean meal-induced intestinal injury in an early-weaned piglet model.Front Vet Sci. 2025 Jun 13;12:1552482. doi: 10.3389/fvets.2025.1552482. eCollection 2025. Front Vet Sci. 2025. PMID: 40586032 Free PMC article.
-
Effects of increasing dietary arginine supply during the three first weeks after weaning on pig growth performance, plasma amino acid concentrations, and health status.Transl Anim Sci. 2024 Apr 1;8:txae047. doi: 10.1093/tas/txae047. eCollection 2024. Transl Anim Sci. 2024. PMID: 38651117 Free PMC article.
-
Anti-Nutritional Factors of Plant Protein Feeds for Ruminants and Methods for Their Elimination.Animals (Basel). 2025 Apr 11;15(8):1107. doi: 10.3390/ani15081107. Animals (Basel). 2025. PMID: 40281941 Free PMC article. Review.
-
Soy-based purified ingredient diet affects mouse gut permeability and the microbiome in fragile X mice.Front Mol Neurosci. 2025 Mar 21;18:1520211. doi: 10.3389/fnmol.2025.1520211. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40190341 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

