Response of Osteoblasts on Amine-Based Nanocoatings Correlates with the Amino Group Density
- PMID: 37764281
- PMCID: PMC10534789
- DOI: 10.3390/molecules28186505
Response of Osteoblasts on Amine-Based Nanocoatings Correlates with the Amino Group Density
Abstract
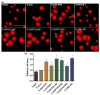
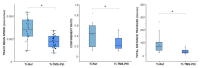
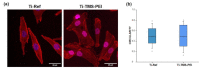
Increased life expectancy in industrialized countries is causing an increased incidence of osteoporosis and the need for bioactive bone implants. The integration of implants can be improved physically, but mainly by chemical modifications of the material surface. It was recognized that amino-group-containing coatings improved cell attachment and intracellular signaling. The aim of this study was to determine the role of the amino group density in this positive cell behavior by developing controlled amino-rich nanolayers. This work used covalent grafting of polymer-based nanocoatings with different amino group densities. Titanium coated with the positively-charged trimethoxysilylpropyl modified poly(ethyleneimine) (Ti-TMS-PEI), which mostly improved cell area after 30 min, possessed the highest amino group density with an N/C of 32%. Interestingly, changes in adhesion-related genes on Ti-TMS-PEI could be seen after 4 h. The mRNA microarray data showed a premature transition of the MG-63 cells into the beginning differentiation phase after 24 h indicating Ti-TMS-PEI as a supportive factor for osseointegration. This amino-rich nanolayer also induced higher bovine serum albumin protein adsorption and caused the cells to migrate slower on the surface after a more extended period of cell settlement as an indication of a better surface anchorage. In conclusion, the cell spreading on amine-based nanocoatings correlated well with the amino group density (N/C).
Keywords: actin cytoskeleton; amino coating; gene expression; material characterization; microarray; polymer; spreading; surface charge; surface modification; wettability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

