NMR-Verified Dearomatization of 5,7-Substituted Pyrazolo[1,5-a]pyrimidines
- PMID: 37764360
- PMCID: PMC10535613
- DOI: 10.3390/molecules28186584
NMR-Verified Dearomatization of 5,7-Substituted Pyrazolo[1,5-a]pyrimidines
Abstract
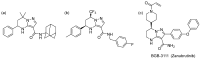
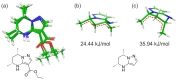
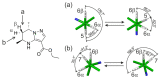
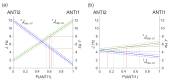
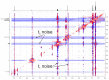
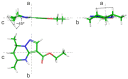
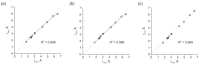
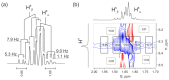
Tetrahydropyrazolo[1,5-a]pyrimidine (THPP) is an attractive scaffold for designing biologically active compounds. The most obvious way to obtain such compounds is to reduce pyrazolopyrimidines with complex hydrides, because the pyrimidine ring is reduced in the preference over the pyrazole ring. The presence of substituents at positions five and seven of pyrazolo[1,5-a]pyrimidines complicates the set of reaction products but makes it more attractive for medicinal chemistry because four possible stereoisomers can be formed during reduction. However, the formation of only syn-isomers has been described in the literature. This article is the first report on the formation of anti-configured isomers along with syn-isomers in the reduction of model 5,7-dimethylpyrazolo[1,5-a]pyrimidine, which was confirmed by NMR. The bicyclic core in the syn-configuration was shown to be conformationally stable, which was used to estimate the long-range interproton distances using NOESY data. At the same time, long-range dipole-dipole interactions corresponding to a distance between protons of more than 6 Å were first registered and quantified. In turn, the bicyclic core in the trans-configuration represents a conformationally labile system. For these structures, an analysis of conformations observed in solutions was carried out. Our results indicate the significant potential of trans-configured tetrahydropyrazolo[1,5-a]pyrimidines for the development of active small molecules. While possessing structural lability due to the low energy of the conformational transition, they have the ability to adjust to the active site of the desired target.
Keywords: NOESY; conformational analysis; dearomatization; long-range interproton distance; proton–proton vicinal constants; pyrazolo[1,5-a]pyrimidine; t1 noise; t2 noise.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Neuhaus D., Williamson M.P. The Nuclear Overhauser Effect in Structural and Conformational Analysis. 2nd ed. Wiley-VCH; Weinheim, Germany: 2000.
-
- Boros S., Gáspári Z., Batta G. Accurate NMR determinations of proton–proton distances. In: Webb G., editor. Annual Reports on NMR Spectroscopy. 1st ed. Volume 94. Academic Press; London, UK: 2018. pp. 1–38.
-
- Contreras R.H., Peralta J.E. Angular dependence of spin-spin coupling constants. Progr. Nucl. Magn. Reson. Spectrosc. 2000;37:321–425. doi: 10.1016/S0079-6565(00)00027-3. - DOI
-
- Karplus M. Vicinal proton coupling in nuclear magnetic resonance. J. Am. Chem. Soc. 1963;85:2870–2871. doi: 10.1021/ja00901a059. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

