Adsorption of Basic Yellow 28 and Basic Blue 3 Dyes from Aqueous Solution Using Silybum Marianum Stem as a Low-Cost Adsorbent
- PMID: 37764414
- PMCID: PMC10536612
- DOI: 10.3390/molecules28186639
Adsorption of Basic Yellow 28 and Basic Blue 3 Dyes from Aqueous Solution Using Silybum Marianum Stem as a Low-Cost Adsorbent
Abstract
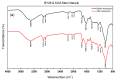
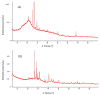
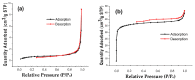
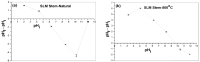
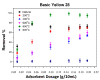
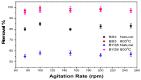
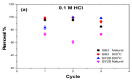
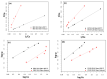
In the present study, the ability of an adsorbent (SLM Stem) obtained from the stem of the Silybum Marianum plant to treat wastewater containing the cationic dyes basic blue 3 (BB3) and basic yellow 28 (BY28) from aqueous solutions was investigated using a batch method. Then, the SLM Stem (SLM Stem-Natural) adsorbent was carbonized at different temperatures (200-900 °C) and the removal capacity of the products obtained for both dyes was examined again. The investigation continued with the product carbonized at 800 °C (SLM Stem-800 °C), the adsorbent with the highest removal capacity. The dyestuff removal studies were continued with the SLM Stem-Natural and SLM Stem-800 °C adsorbents because they had the highest removal values. The surface properties of these two adsorbents were investigated using IR, SEM, and XRD measurements. It was determined that the SLM Stem-Natural has mainly non-porous material, and the SLM Stem-800 °C has a microporous structure. The optimal values for various parameters, including adsorbent amount, initial dye solution concentration, contact time, temperature, pH, and agitation speed, were investigated for BY28 dye and were 0.05 g, 15 mg/L, 30 min, 40 °C, pH 6 and 100 rpm when SLM Stem-Natural adsorbent was used and, 0.15 g, 30 mg/L, 30 min, 40 °C, pH 10, and 150 rpm when SLM Stem-800 °C adsorbent was used. For BB3 dye, optimal parameter values of 0.20 g, 10 mg/L, 30 min, 25 °C, pH 7, and 100 rpm were obtained when SLM Stem-Natural adsorbent was used and 0.15 g, 15 mg/L, 40 min, 40 °C, pH 10, and 100 rpm when SLM Stem-800 °C adsorbent was used. The Langmuir isotherm described the adsorption process best, with a value of r2 = 0.9987. When SLM Stem-800 °C adsorbent was used for BY28 dye at 25 °C, the highest qm value in the Langmuir isotherm was 271.73 mg/g. When the study was repeated with actual water samples under optimum conditions, the highest removal for the BY28 dye was 99.9% in tap water with the SLM Stem-800 °C adsorbent. Furthermore, the reuse study showed the adsorbent's efficiency even after three repetitions.
Keywords: adsorption; basic blue 3; basic yellow 28; silybum marianum stem.
Conflict of interest statement
The author declares no conflict of interest. The author affirms that no competitive financial interests or personal relationships could have affected the reported work.
Figures













References
-
- Ojedabenitez S., Beraudlozano J. The municipal solid waste cycle in Mexico: Final disposal. Resour. Conserv. Recycl. 2003;39:239–250. doi: 10.1016/s0921-3449(03)00030-2. - DOI
-
- Nagano S., Tamon H., Adzumi T., Nakagawa K., Suzuki T. Activated carbon from municipal waste. Carbon. 2000;38:915–920. doi: 10.1016/s0008-6223(99)00208-0. - DOI
-
- Montagnaro F., Santoro L. Reuse of coal combustion ashes as dyes and heavy metal adsorbents: Effect of sieving and demineralization on waste properties and adsorption capacity. Chem. Eng. J. 2009;150:174–180. doi: 10.1016/j.cej.2008.12.022. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

