Palladium Nanoparticles Grafted onto Phytochemical Functionalized Biochar: A Sustainable Nanozyme for Colorimetric Sensing of Glucose and Glutathione
- PMID: 37764452
- PMCID: PMC10537334
- DOI: 10.3390/molecules28186676
Palladium Nanoparticles Grafted onto Phytochemical Functionalized Biochar: A Sustainable Nanozyme for Colorimetric Sensing of Glucose and Glutathione
Abstract
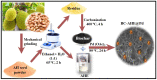
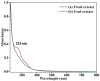
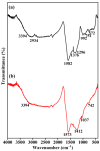
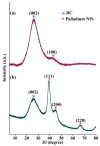
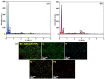
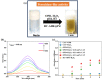
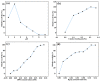
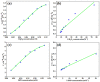
The devising and development of numerous enzyme mimics, particularly nanoparticles and nanomaterials (nanozymes), have been sparked by the inherent limitations imposed by natural enzymes. Peroxidase is one of the enzymes that is extensively utilized in commercial, medical, and biological applications because of its outstanding substrate selectivity. Herein, we present palladium nanoparticles grafted on Artocarpus heterophyllus (jackfruit) seed-derived biochar (BC-AHE@Pd) as a novel nanozyme to imitate peroxidase activity en route to the rapid and colorimetric detection of H2O2, exploiting o-phenylenediamine as a peroxidase substrate. The biogenically generated BC-AHE@Pd nanocatalyst was synthesized utilizing Artocarpus heterophyllus seed extract as the reducing agent for nanoparticle formation, while the residue became the source for biochar. Various analytical techniques like FT-IR, GC-MS, FE-SEM, EDS, TEM, SAED pattern, p-XRD, and ICP-OES, were used to characterize the BC-AHE@Pd nanocatalyst. The intrinsic peroxidase-like activity of the BC-AHE@Pd nanocatalyst was extended as a prospective nanosensor for the estimation of the biomolecules glucose and glutathione. Moreover, the BC-AHE@Pd nanocatalyst showed recyclability up to three recycles without any significant loss in activity.
Keywords: BC-AHE@Pd; H2O2 sensing; biochar; glucose; glutathione; nanozyme.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- Linsinger T.P., Roebben G., Solans C., Ramsch R. Reference materials for measuring the size of nanoparticles. TrAC Trends Anal. Chem. 2011;30:18–27. doi: 10.1016/j.trac.2010.09.005. - DOI
-
- Ghosh S.K., Pal T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007;107:4797–4862. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

