Construction and Evaluation of Alginate Dialdehyde Grafted RGD Derivatives/Polyvinyl Alcohol/Cellulose Nanocrystals IPN Composite Hydrogels
- PMID: 37764467
- PMCID: PMC10534451
- DOI: 10.3390/molecules28186692
Construction and Evaluation of Alginate Dialdehyde Grafted RGD Derivatives/Polyvinyl Alcohol/Cellulose Nanocrystals IPN Composite Hydrogels
Abstract
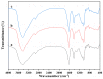
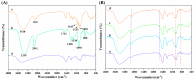
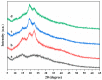
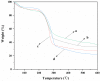
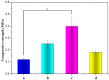
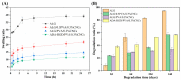
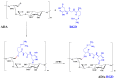
To enhance the mechanical strength and cell adhesion of alginate hydrogel, making it satisfy the requirements of an ideal tissue engineering scaffold, the grafting of Arg-Gly-Asp (RGD) polypeptide sequence onto the alginate molecular chain was conducted by oxidation of sodium periodate and subsequent reduction amination of 2-methylpyridine borane complex (2-PBC) to synthesize alginate dialdehyde grafted RGD derivatives (ADA-RGD) with good cellular affinity. The interpenetrating network (IPN) composite hydrogels of alginate/polyvinyl alcohol/cellulose nanocrystals (ALG/PVA/CNCs) were fabricated through a physical mixture of ion cross-linking of sodium alginate (SA) with hydroxyapatite/D-glucono-δ-lactone (HAP/GDL), and physical cross-linking of polyvinyl alcohol (PVA) by a freezing/thawing method, using cellulose nanocrystals (CNCs) as the reinforcement agent. The effects of the addition of CNCs and different contents of PVA on the morphology, thermal stability, mechanical properties, swelling, biodegradability, and cell compatibility of the IPN composite hydrogels were investigated, and the effect of RGD grafting on the biological properties of the IPN composite hydrogels was also studied. The resultant IPN ALG/PVA/CNCs composite hydrogels exhibited good pore structure and regular 3D morphology, whose pore size and porosity could be regulated by adjusting PVA content and the addition of CNCs. By increasing the PVA content, the number of physical cross-linking points in PVA increased, resulting in greater stress support for the IPN composite hydrogels of ALG/PVA/CNCs and consequently improving their mechanical characteristics. The creation of the IPN ALG/PVA/CNCs composite hydrogels' physical cross-linking network through intramolecular or intermolecular hydrogen bonding led to improved thermal resistance and reduced swelling and biodegradation rate. Conversely, the ADA-RGD/PVA/CNCs IPN composite hydrogels exhibited a quicker degradation rate, attributed to the elimination of ADA-RGD by alkali. The results of the in vitro cytocompatibility showed that ALG/0.5PVA/0.3%CNCs and ADA-RGD/PVA/0.3%CNCs composite hydrogels showed better proliferative activity in comparison with other composite hydrogels, while ALG/PVA/0.3%CNCs and ADA-RGD/PVA/0.3%CNCs composite hydrogels displayed obvious proliferation effects, indicating that PVA, CNCs, and ADA-RGD with good biocompatibility were conducive to cell proliferation and differentiation for the IPN composite hydrogels.
Keywords: alginate; cellular adhesion; interpenetrating network composite hydrogels; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Fabrication and Evaluation of Alginate/Bacterial Cellulose Nanocrystals-Chitosan-Gelatin Composite Scaffolds.Molecules. 2021 Aug 18;26(16):5003. doi: 10.3390/molecules26165003. Molecules. 2021. PMID: 34443588 Free PMC article.
-
Dodecyl glycoside intercalated organo-montmorillonite promoted biomimetic alginate/microcrystalline cellulose/nano-hydroxyapatite composite hydrogels for bone tissue engineering.Int J Biol Macromol. 2025 May;310(Pt 2):143304. doi: 10.1016/j.ijbiomac.2025.143304. Epub 2025 Apr 17. Int J Biol Macromol. 2025. PMID: 40253035
-
Interpenetrating and semi-interpenetrating network superabsorbent hydrogels based on sodium alginate and cellulose nanocrystals: A biodegradable and high-performance solution for adult incontinence pads.Int J Biol Macromol. 2023 Dec 31;253(Pt 8):127118. doi: 10.1016/j.ijbiomac.2023.127118. Epub 2023 Oct 4. Int J Biol Macromol. 2023. PMID: 37802434
-
A sodium alginate-based sustained-release IPN hydrogel and its applications.RSC Adv. 2020 Oct 30;10(65):39722-39730. doi: 10.1039/d0ra04316h. eCollection 2020 Oct 27. RSC Adv. 2020. PMID: 35515393 Free PMC article. Review.
-
Cellulose-alginate hydrogels and their nanocomposites for water remediation and biomedical applications.Environ Res. 2024 Feb 15;243:117889. doi: 10.1016/j.envres.2023.117889. Epub 2023 Dec 10. Environ Res. 2024. PMID: 38086501 Review.
Cited by
-
Hydrogel-Based 3D Bioprinting Technology for Articular Cartilage Regenerative Engineering.Gels. 2024 Jun 28;10(7):430. doi: 10.3390/gels10070430. Gels. 2024. PMID: 39057453 Free PMC article. Review.
-
Excellent Dark/Light Dual-Mode Photoresponsive Activities Based on g-C3N4/CMCh/PVA Nanocomposite Hydrogel Using Electron Beam Radiation Method.Molecules. 2023 Nov 11;28(22):7544. doi: 10.3390/molecules28227544. Molecules. 2023. PMID: 38005263 Free PMC article.
-
Hydrogel composite scaffold repairs knee cartilage defects: a systematic review.RSC Adv. 2025 Apr 8;15(13):10337-10364. doi: 10.1039/d5ra01031d. eCollection 2025 Mar 28. RSC Adv. 2025. PMID: 40200956 Free PMC article. Review.
References
-
- Collins M.N., Ren G., Young K., Pina S., Reis R.L., Oliveira J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021;31:2010609. doi: 10.1002/adfm.202010609. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

