Metformin-Loaded Chitosan Hydrogels Suppress Bladder Tumor Growth in an Orthotopic Mouse Model via Intravesical Administration
- PMID: 37764495
- PMCID: PMC10534355
- DOI: 10.3390/molecules28186720
Metformin-Loaded Chitosan Hydrogels Suppress Bladder Tumor Growth in an Orthotopic Mouse Model via Intravesical Administration
Abstract
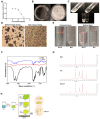
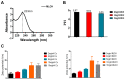
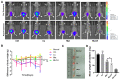
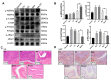
Our previous study found that the intravesical perfusion of metformin has excellent inhibitory effects against bladder cancer (BC). However, this administration route allows the drug to be diluted and excreted in urine. Therefore, increasing the adhesion of metformin to the bladder mucosal layer may prolong the retention time and increase the pharmacological activity. It is well known that chitosan (Cs) has a strong adhesion to the bladder mucosal layer. Thus, this study established a novel formulation of metformin to enhance its antitumor activity by extending its retention time. In this research, we prepared Cs freeze-dried powder and investigated the effect of metformin-loaded chitosan hydrogels (MLCH) in vitro and in vivo. The results showed that MLCH had a strong inhibitory effect against proliferation and colony formation in vitro. The reduction in BC weight and the expression of tumor biomarkers in orthotopic mice showed the robust antitumor activity of MLCH via intravesical administration in vivo. The non-toxic profile of MLCH was observed as well, using histological examinations. Mechanistically, MLCH showed stronger functional activation of the AMPKα/mTOR signaling pathway compared with metformin alone. These findings aim to make this novel formulation an efficient candidate for managing BC via intravesical administration.
Keywords: bladder cancer; chitosan hydrogels; intravesical administration; metformin; orthotopic model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
High efficacy of intravesical treatment of metformin on bladder cancer in preclinical model.Oncotarget. 2016 Feb 23;7(8):9102-17. doi: 10.18632/oncotarget.6933. Oncotarget. 2016. PMID: 26802022 Free PMC article.
-
Design and evaluation of an intravesical delivery system for superficial bladder cancer: preparation of gemcitabine HCl-loaded chitosan-thioglycolic acid nanoparticles and comparison of chitosan/poloxamer gels as carriers.Int J Nanomedicine. 2015 Oct 14;10:6493-507. doi: 10.2147/IJN.S93750. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26508855 Free PMC article.
-
Intravesical delivery of rapamycin via folate-modified liposomes dispersed in thermo-reversible hydrogel.Int J Nanomedicine. 2019 Aug 5;14:6249-6268. doi: 10.2147/IJN.S216432. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31496684 Free PMC article.
-
Current status of the development of intravesical drug delivery systems for the treatment of bladder cancer.Expert Opin Drug Deliv. 2020 Nov;17(11):1555-1572. doi: 10.1080/17425247.2020.1810016. Epub 2020 Sep 4. Expert Opin Drug Deliv. 2020. PMID: 32791923 Review.
-
Innovative applications of natural polysaccharide polymers in intravesical therapy of bladder diseases.Carbohydr Polym. 2025 Apr 15;354:123307. doi: 10.1016/j.carbpol.2025.123307. Epub 2025 Jan 22. Carbohydr Polym. 2025. PMID: 39978897 Review.
Cited by
-
Inhibition of Cyclin D1 by Novel Biguanide Derivative YB-004 Increases the Sensitivity of Bladder Cancer to Olaparib via Causing G0 / G1 Arrest.Int J Biol Sci. 2025 Feb 18;21(5):1984-1998. doi: 10.7150/ijbs.105072. eCollection 2025. Int J Biol Sci. 2025. PMID: 40083696 Free PMC article.
References
-
- Xiao D., Hu X., Peng M., Deng J., Zhou S., Xu S., Wu J., Yang X. Inhibitory role of proguanil on the growth of bladder cancer via enhancing EGFR degradation and inhibiting its downstream signaling pathway to induce autophagy. Cell Death Dis. 2022;13:499. doi: 10.1038/s41419-022-04937-z. - DOI - PMC - PubMed
-
- Kitson S.J., Maskell Z., Sivalingam V.N., Allen J.L., Ali S., Burns S., Gilmour K., Latheef R., Slade R.J., Pemberton P.W., et al. PRE-surgical Metformin In Uterine Malignancy (PREMIUM): A Multi-Center, Randomized Double-Blind, Placebo-Controlled Phase III Trial. Clin. Cancer Res. 2019;25:2424–2432. doi: 10.1158/1078-0432.CCR-18-3339. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82172653/The National Natural Science Foundation of China
- KF2022001, KF2021017/Institutional Open Fund
- 2022XKQ0205/Key Project of Developmental Biology and Breeding from Hunan Province
- kq2202387/Natural Science Foundation of Changsha
- QL20220115/Graduate Scientific Research Innovation Project of Hunan Province, China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

