Extractive Spectrophotometric Determination and Theoretical Investigations of Two New Vanadium(V) Complexes
- PMID: 37764499
- PMCID: PMC10536437
- DOI: 10.3390/molecules28186723
Extractive Spectrophotometric Determination and Theoretical Investigations of Two New Vanadium(V) Complexes
Abstract
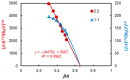
Two new vanadium (V) complexes involving 6-hexyl-4-(2-thiazolylazo)resorcinol (HTAR) and tetrazolium cation were studied. The following commercially available tetrazolium salts were used as the cation source: tetrazolium red (2,3,5-triphenyltetrazol-2-ium;chloride, TTC) and neotetrazolium chloride (2-[4-[4-(3,5-diphenyltetrazol-2-ium-2-yl)phenyl]phenyl]-3,5-diphenyltetrazol-2-ium;dichloride, NTC). The cations (abbreviated as TT+ and NTC+) impart high hydrophobicity to the ternary complexes, allowing vanadium to be easily extracted and preconcentrated in one step. The complexes have different stoichiometry. The V(V)-HTAR-TTC complex dimerizes in the organic phase (chloroform) and can be represented by the formula [(TT+)[VO2(HTAR)]]2. The other complex is monomeric (NTC+)[VO2(HTAR)]. The cation has a +1 charge because one of the two chloride ions remains undissociated: NTC+ = (NT2+Cl-)+. The ground-state equilibrium geometries of the constituent cations and final complexes were optimized at the B3LYP and HF levels of theory. The dimer [(TT+)[VO2(HTAR)]]2 is more suitable for practical applications due to its better extraction characteristics and wider pH interval of formation and extraction. It was used for cheap and reliable extraction-spectrophotometric determination of V(V) traces in real samples. The absorption maximum, molar absorptivity coefficient, limit of detection, and linear working range were 549 nm, 5.2 × 104 L mol-1 cm-1, 4.6 ng mL-1, and 0.015-2.0 μg mL-1, respectively.
Keywords: 6-hexyl-4-(2-thiazolylazo)resorcinol; HF and B3LYP calculations; liquid–liquid extraction; spectrophotometric determination; tetrazolium; vanadium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














References
-
- Rudnick R.L., Gao S. In: Treatise on Geochemistry. 2nd ed. Holland H.D., Turekian K.K., editors. Volume 4. Elsevier; Oxford, UK: 2014. pp. 1–51. - DOI
-
- Roychoudhury A. Vanadium uptake and toxicity in plants. SF J. Agri. Crop Manag. 2020;1:1010.
-
- Hope B.K. A dynamic model for the global cycling of anthropogenic vanadium. Glob. Biogeochem. Cycles. 2008;22:GB4021. doi: 10.1029/2008GB003283. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

