Myrcene: A Natural Compound Showing Anticancer Activity in HeLa Cells
- PMID: 37764505
- PMCID: PMC10537210
- DOI: 10.3390/molecules28186728
Myrcene: A Natural Compound Showing Anticancer Activity in HeLa Cells
Abstract
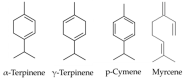
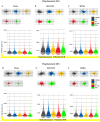
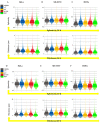
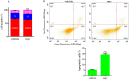
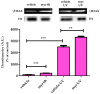
γ-terpinene, α-terpinene, p-cymene, and myrcene are monoterpenes found in many essential oils extracted from a variety of plants and spices. Myrcene also occurs naturally in plants such as hops, cannabis, lemongrass, and verbena and is used as a flavoring agent in food and beverage manufacturing. In this research, the biological efficacy of γ-terpinene, α-terpinene, p-cymene, and myrcene was studied in human cell lines (HeLa, SH-SY5Y, and HDFa). Cytotoxicity, cell proliferation, cell migration, and morphology assays were performed to obtain detailed information on the anticancer properties. Our results show that myrcene has potential biological activity, especially in HeLa cells. In this cell line, it leads to an arrest of proliferation, a decrease in motility and morphological changes with loss of sphericity and thickness, and DNA damage. In addition, the interaction of γ-terpinene, α-terpinene, p-terpinene, and myrcene with calf thymus DNA (ct-DNA) was studied by UV-visible spectrophotometry. DNA binding experiments show that only myrcene can interact with DNA with an apparent dissociation constant (Kd) of 29 × 10-6 M.
Keywords: cancer; cell cycle; monoterpenes; myrcene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

