The Potentiating Effect of Graphene Oxide on the Arylhydrocarbon Receptor (AhR)-Cytochrome P4501A (Cyp1A) System Activated by Benzo(k)fluoranthene (BkF) in Rainbow Trout Cell Line
- PMID: 37764529
- PMCID: PMC10534689
- DOI: 10.3390/nano13182501
The Potentiating Effect of Graphene Oxide on the Arylhydrocarbon Receptor (AhR)-Cytochrome P4501A (Cyp1A) System Activated by Benzo(k)fluoranthene (BkF) in Rainbow Trout Cell Line
Abstract
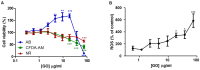
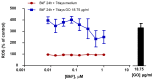
The increasing use of graphene oxide (GO) will result in its release into the environment; therefore, it is essential to determine its final fate and possible metabolism by organisms. The objective of this study was to assess the possible role of the aryl hydrocarbon receptor (AhR)-dependent cytochrome P4501A (Cyp1A) detoxification activities on the catabolism of GO. Our hypothesis is that GO cannot initially interact with the AhR, but that after an initial degradation caused by other mechanisms, small fractions of GO could activate the AhR, inducing Cyp1A. The environmental pollutant benzo(k)fluoranthene (BkF) was used for the initial activation of the AhR in the rainbow trout (Oncorhynchus mykiss) cell line RTL-W1. Pre-, co-, and post-exposure experiments with GO were performed and Cyp1A induction was monitored. The strong stimulation of Cyp1A observed in cells after exposure to GO, when BkF levels were not detected in the system, suggests a direct action of GO. The role of the AhR was confirmed by a blockage of the observed effects in co-treatment experiments with αNF (an AhR antagonist). These results suggest a possible role for the AhR and Cyp1A system in the cellular metabolism of GO and that GO could modulate the toxicity of environmental pollutants.
Keywords: Cyp1A; EROD; aryl hydrocarbon receptor; fish; graphene.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



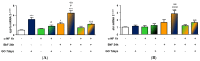
References
-
- Joshi K., Mazumder B., Chattopadhya P., Bora N.S., Goyary D., Karmakar S. Graphene family of nanomaterials: Reviewing advanced applications in drug delivery and medicine. Curr. Drug Deliv. 2019;16:195–214. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

