The Apoptosis Inhibitor Protein Survivin Is a Critical Cytoprotective Resistor against Silica-Based Nanotoxicity
- PMID: 37764575
- PMCID: PMC10535920
- DOI: 10.3390/nano13182546
The Apoptosis Inhibitor Protein Survivin Is a Critical Cytoprotective Resistor against Silica-Based Nanotoxicity
Abstract
Exposure to nanoparticles is inevitable as they become widely used in industry, cosmetics, and foods. However, knowledge of their (patho)physiological effects on biological entry routes of the human body and their underlying molecular mechanisms is still fragmented. Here, we examined the molecular effects of amorphous silica nanoparticles (aSiNPs) on cell lines mimicking the alveolar-capillary barrier of the lung. After state-of-the-art characterization of the used aSiNPs and the cell model, we performed cell viability-based assays and a protein analysis to determine the aSiNP-induced cell toxicity and underlying signaling mechanisms. We revealed that aSiNPs induce apoptosis in a dose-, time-, and size-dependent manner. aSiNP-induced toxicity involves the inhibition of pro-survival pathways, such as PI3K/AKT and ERK signaling, correlating with reduced expression of the anti-apoptotic protein Survivin on the protein and transcriptional levels. Furthermore, induced Survivin overexpression mediated resistance against aSiNP-toxicity. Thus, we present the first experimental evidence suggesting Survivin as a critical cytoprotective resistor against silica-based nanotoxicity, which may also play a role in responses to other NPs. Although Survivin's relevance as a biomarker for nanotoxicity needs to be demonstrated in vivo, our data give general impetus to investigate the pharmacological modulation of Survivin`s functions to attenuate the harmful effects of acute or chronic inhalative NP exposure.
Keywords: alveolar-capillary barrier; amorphous silica nanoparticles; cytotoxic response; inflammation; lung model; nanotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

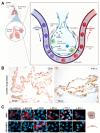


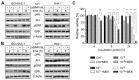

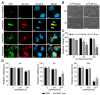

References
-
- Davies J.C.T. PEN 13-Nanotechnology Oversight. [(accessed on 14 January 2019)]. Available online: https://www.wilsoncenter.org/publication/pen-13-nanotechnology-oversight.
-
- Liljenström C.L.D., Finnveden G. Silicon-Based Nanomaterials in a Life-Cycle Perspective, Including a Case Study on Self-Cleaning Coatings. 2013. [(accessed on 14 January 2019)]. Available online: https://www.researchgate.net/publication/280264076_Silicon-based_nanomat....
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

