Revealing the Adsorption Mechanisms of Methanol on Lithium-Doped Porous Carbon through Experimental and Theoretical Calculations
- PMID: 37764593
- PMCID: PMC10537878
- DOI: 10.3390/nano13182564
Revealing the Adsorption Mechanisms of Methanol on Lithium-Doped Porous Carbon through Experimental and Theoretical Calculations
Abstract
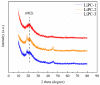
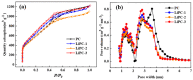
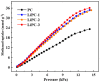
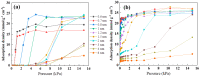
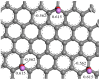
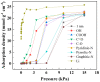
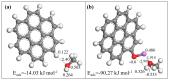
Previous reports have shown that it is difficult to improve the methanol adsorption performance of nitrogen and oxygen groups due to their low polarity. Here, we first prepared porous carbon with a high specific surface area and large pore volume using benzimidazole as a carbon precursor and KOH as an activating agent. Then, we improved the surface polarity of the porous carbon by doping with Lithium (Li) to enhance the methanol adsorption performance. The results showed that the methanol adsorption capacity of Li-doped porous carbon reached 35.4 mmol g-1, which increased by 57% compared to undoped porous carbon. Molecular simulation results showed that Li doping not only improved the methanol adsorption performance at low pressure, but also at relatively high pressure. This is mainly because Li-modified porous carbon has higher surface polarity than nitrogen and oxygen-modified surfaces, which can generate stronger electrostatic interactions. Furthermore, through density functional theory (DFT) calculations, we determined the adsorption energy, adsorption distance, and charge transfer between Li atom and methanol. Our results demonstrate that Li doping enhances the adsorption energy, reduces the adsorption distance, and increases the charge transfer in porous carbon. The mechanism of methanol adsorption by Li groups was revealed through experimental and theoretical calculations, providing a theoretical basis for the design and preparation of methanol adsorbents.
Keywords: adsorption mechanism; lithium doping; methanol adsorption; pore structure; porous carbon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Targeted improvement of narrow micropores in porous carbon for enhancing trace benzene vapor removal: Revealing the adsorption mechanism via experimental and molecular simulation.J Colloid Interface Sci. 2024 Oct;671:770-778. doi: 10.1016/j.jcis.2024.05.165. Epub 2024 May 23. J Colloid Interface Sci. 2024. PMID: 38830289
-
New insights into NO adsorption on alkali metal and transition metal doped graphene nanoribbon surface: A DFT approach.J Mol Graph Model. 2022 Mar;111:108109. doi: 10.1016/j.jmgm.2021.108109. Epub 2021 Dec 16. J Mol Graph Model. 2022. PMID: 34952481
-
Porous Carbon Nanofibers with Heteroatoms Doped by Electrospinning Exhibit Excellent Acetone and Carbon Dioxide Adsorption Performance: The Contributions of Pore Structure and Functional Groups.ACS Omega. 2021 Nov 4;6(45):30716-30725. doi: 10.1021/acsomega.1c04618. eCollection 2021 Nov 16. ACS Omega. 2021. PMID: 34805699 Free PMC article.
-
Adsorption of Volatile Organic Compounds (VOCs) on Oxygen-rich Porous Carbon Materials Obtained from Glucose/Potassium Oxalate.Chem Asian J. 2021 May 3;16(9):1118-1129. doi: 10.1002/asia.202100098. Epub 2021 Mar 30. Chem Asian J. 2021. PMID: 33725405
-
Carbon Nanostructures Doped with Transition Metals for Pollutant Gas Adsorption Systems.Molecules. 2021 Sep 2;26(17):5346. doi: 10.3390/molecules26175346. Molecules. 2021. PMID: 34500783 Free PMC article. Review.
References
-
- Chakraborty R., Vilya K., Pradhan M., Nayak A.K. Recent advancement of biomass-derived porous carbon based materials for energy and environmental remediation applications. J. Mater. Chem. A. 2022;10:6965–7005. doi: 10.1039/D1TA10269A. - DOI
-
- Liu Z., Kai Z., Ying W., Xi H. New functionalized IRMOF-10 with strong affinity for methanol: A simulation study. Appl. Surf. Sci. 2018;440:351–358. doi: 10.1016/j.apsusc.2018.01.028. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

