A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies
- PMID: 37764614
- PMCID: PMC10536909
- DOI: 10.3390/nano13182585
A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies
Abstract
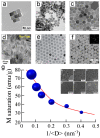
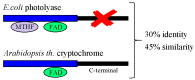
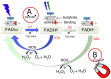
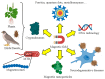
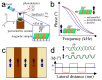
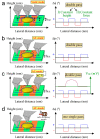
Magnetism plays a pivotal role in many biological systems. However, the intensity of the magnetic forces exerted between magnetic bodies is usually low, which demands the development of ultra-sensitivity tools for proper sensing. In this framework, magnetic force microscopy (MFM) offers excellent lateral resolution and the possibility of conducting single-molecule studies like other single-probe microscopy (SPM) techniques. This comprehensive review attempts to describe the paramount importance of magnetic forces for biological applications by highlighting MFM's main advantages but also intrinsic limitations. While the working principles are described in depth, the article also focuses on novel micro- and nanofabrication procedures for MFM tips, which enhance the magnetic response signal of tested biomaterials compared to commercial nanoprobes. This work also depicts some relevant examples where MFM can quantitatively assess the magnetic performance of nanomaterials involved in biological systems, including magnetotactic bacteria, cryptochrome flavoproteins, and magnetic nanoparticles that can interact with animal tissues. Additionally, the most promising perspectives in this field are highlighted to make the reader aware of upcoming challenges when aiming toward quantum technologies.
Keywords: atomic force microscopy; biological systems; drug delivery; magnetic force microscopy; magnetic properties; magnetic tip fabrication; nanofabrication; quantum technologies; single-molecule studies.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Magnetic force microscopy: quantitative issues in biomaterials.Biomatter. 2014;4:e29507. doi: 10.4161/biom.29507. Epub 2014 Jul 22. Biomatter. 2014. PMID: 25050758 Free PMC article.
-
Customized MFM probes with high lateral resolution.Beilstein J Nanotechnol. 2016 Jul 25;7:1068-74. doi: 10.3762/bjnano.7.100. eCollection 2016. Beilstein J Nanotechnol. 2016. PMID: 27547625 Free PMC article.
-
Accurate determination of MFM tip's magnetic parameters on nanoparticles by decoupling the influence of electrostatic force.Nanotechnology. 2022 Aug 31;33(47). doi: 10.1088/1361-6528/ac8998. Nanotechnology. 2022. PMID: 35970138
-
Identification of nanoparticles and nanosystems in biological matrices with scanning probe microscopy.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018 Nov;10(6):e1521. doi: 10.1002/wnan.1521. Epub 2018 Apr 17. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018. PMID: 29665287 Review.
-
Magnetic Nanotweezers for Interrogating Biological Processes in Space and Time.Acc Chem Res. 2018 Apr 17;51(4):839-849. doi: 10.1021/acs.accounts.8b00004. Epub 2018 Mar 28. Acc Chem Res. 2018. PMID: 29589897 Free PMC article. Review.
Cited by
-
Nanomaterial-based cancer immunotherapy: enhancing treatment strategies.Front Chem. 2024 Oct 10;12:1492215. doi: 10.3389/fchem.2024.1492215. eCollection 2024. Front Chem. 2024. PMID: 39449695 Free PMC article. Review.
-
Easy One-Pot Decoration of Graphene Oxide Nanosheets by Green Silver Nanoparticles.Int J Mol Sci. 2025 Jan 16;26(2):713. doi: 10.3390/ijms26020713. Int J Mol Sci. 2025. PMID: 39859427 Free PMC article.
-
Improving the Efficacy of Common Cancer Treatments via Targeted Therapeutics towards the Tumour and Its Microenvironment.Pharmaceutics. 2024 Jan 26;16(2):175. doi: 10.3390/pharmaceutics16020175. Pharmaceutics. 2024. PMID: 38399237 Free PMC article. Review.
-
Multiparametric Magnetic Resonance Imaging of Penile Cancer: A Pictorial Review.Cancers (Basel). 2023 Nov 8;15(22):5324. doi: 10.3390/cancers15225324. Cancers (Basel). 2023. PMID: 38001583 Free PMC article. Review.
-
Carbon Nanodot-Microbe-Plant Nexus in Agroecosystem and Antimicrobial Applications.Nanomaterials (Basel). 2024 Jul 25;14(15):1249. doi: 10.3390/nano14151249. Nanomaterials (Basel). 2024. PMID: 39120354 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

