The Epigenetic Legacy of Maternal Protein Restriction: Renal Ptger1 DNA Methylation Changes in Hypertensive Rat Offspring
- PMID: 37764741
- PMCID: PMC10535296
- DOI: 10.3390/nu15183957
The Epigenetic Legacy of Maternal Protein Restriction: Renal Ptger1 DNA Methylation Changes in Hypertensive Rat Offspring
Abstract
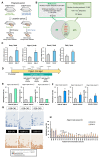
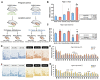
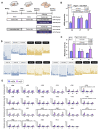
Nutrient imbalances during gestation are a risk factor for hypertension in offspring. Although the effects of prenatal nutritional deficiency on the development of hypertension and cardiovascular diseases in adulthood have been extensively documented, its underlying mechanisms remain poorly understood. In this study, we aimed to elucidate the precise role and functional significance of epigenetic modifications in the pathogenesis of hypertension. To this end, we integrated methylome and transcriptome data to identify potential salt-sensitive hypertension genes using the kidneys of stroke-prone spontaneously hypertensive rat (SHRSP) pups exposed to a low-protein diet throughout their fetal life. Maternal protein restriction during gestation led to a positive correlation between DNA hypermethylation of the renal prostaglandin E receptor 1 (Ptger1) CpG island and high mRNA expression of Ptger1 in offspring, which is consistently conserved. Furthermore, post-weaning low-protein or high-protein diets modified the Ptger1 DNA hypermethylation caused by fetal malnutrition. Here, we show that this epigenetic variation in Ptger1 is linked to disease susceptibility established during fetal stages and could be reprogrammed by manipulating the postnatal diet. Thus, our findings clarify the developmental origins connecting the maternal nutritional environment and potential epigenetic biomarkers for offspring hypertension. These findings shed light on hypertension prevention and prospective therapeutic strategies.
Keywords: DNA methylation; Ptger1; epigenetics; hypertension; kidney; low protein diet; maternal nutrition; nutrigenomics; offspring; postnatal nutrition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Maternal Protein Restriction Alters the Renal Ptger1 DNA Methylation State in SHRSP Offspring.Nutrients. 2018 Oct 5;10(10):1436. doi: 10.3390/nu10101436. Nutrients. 2018. PMID: 30301128 Free PMC article.
-
Postnatal nutrition environment reprograms renal DNA methylation patterns in offspring of maternal protein-restricted stroke-prone spontaneously hypertensive rats.Front Nutr. 2023 Apr 13;10:1134955. doi: 10.3389/fnut.2023.1134955. eCollection 2023. Front Nutr. 2023. PMID: 37125041 Free PMC article.
-
Maternal overnutrition programs epigenetic changes in the regulatory regions of hypothalamic Pomc in the offspring of rats.Int J Obes (Lond). 2018 Aug;42(8):1431-1444. doi: 10.1038/s41366-018-0094-1. Epub 2018 May 17. Int J Obes (Lond). 2018. PMID: 29777232 Free PMC article.
-
Nutrigenetic and Epigenetic Mechanisms of Maternal Nutrition-Induced Glucolipid Metabolism Changes in the Offspring.Nutr Rev. 2025 Apr 1;83(4):728-748. doi: 10.1093/nutrit/nuae048. Nutr Rev. 2025. PMID: 38781288 Review.
-
Interplay between maternal nutrition and epigenetic programming on offspring hypertension.J Nutr Biochem. 2024 May;127:109604. doi: 10.1016/j.jnutbio.2024.109604. Epub 2024 Feb 18. J Nutr Biochem. 2024. PMID: 38373508 Review.
Cited by
-
Unraveling the Role of miR-200b-3p in Attention-Deficit/Hyperactivity Disorder (ADHD) and Its Therapeutic Potential in Spontaneously Hypertensive Rats (SHR).Biomedicines. 2024 Jan 10;12(1):144. doi: 10.3390/biomedicines12010144. Biomedicines. 2024. PMID: 38255250 Free PMC article.
-
Role of DNA methylation transferase in urinary system diseases: From basic to clinical perspectives (Review).Int J Mol Med. 2025 Feb;55(2):19. doi: 10.3892/ijmm.2024.5460. Epub 2024 Nov 22. Int J Mol Med. 2025. PMID: 39575487 Free PMC article. Review.
References
-
- Marshall N.E., Abrams B., Barbour L.A., Catalano P., Christian P., Friedman J.E., Hay W.W., Jr., Hernandez T.L., Krebs N.F., Oken E., et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am. J. Obstet. Gynecol. 2022;226:607–632. doi: 10.1016/j.ajog.2021.12.035. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

