Main Cardiac Histopathologic Alterations in the Acute Phase of Trypanosoma cruzi Infection in a Murine Model
- PMID: 37764892
- PMCID: PMC10534729
- DOI: 10.3390/pathogens12091084
Main Cardiac Histopathologic Alterations in the Acute Phase of Trypanosoma cruzi Infection in a Murine Model
Abstract
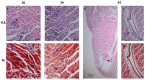
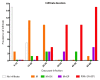
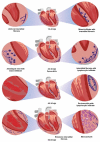
Symptoms in the acute phase of Chagas disease are usually mild and nonspecific. However, after several years, severe complications like dilated heart failure and even death may arise in the chronic phase. Due to the lack of specific symptoms in the acute phase, the aim of this work was to describe and analyze the cardiac histopathology during this phase in a CD1 mouse model by assessing parasitism, fibrotic damage, and the presence and composition of a cellular infiltrate, to determine its involvement in the pathogenesis of lesions in the cardiac tissue. Our results indicate that the acute phase lasts about 62 days post-infection (dpi). A significant increase in parasitemia was observed since 15 dpi, reaching a maximum at 33 dpi (4.1 × 106). The presence of amastigote nests was observed at 15-62 dpi, with a maximum count of 27 nests at 35 dpi. An infiltrate consisting primarily of macrophages and neutrophils was found in the cardiac tissue within the first 30 days, but the abundance of lymphocytes showed an 8 ≥ fold increase at 40-62 dpi. Unifocal interstitial fibrosis was identified after 9 dpi, which subsequently showed a 16 ≥ fold increase at 40-60 dpi, along with a 50% mortality rate in the model under study. The increased area of fibrotic lesions revealed progression in the extent of fibrosis, mainly at 50-62 dpi. The presence of perivasculitis and thrombus circulation disorders was seen in the last days (62 dpi); finally, cases of myocytolysis were observed at 50 and 62 dpi. These histopathological alterations, combined with collagen deposition, seem to lead to the development of interstitial fibrosis and damage to the cardiac tissue during the acute phase of infection. This study provides a more complete understanding of the patterns of histopathological abnormalities involved in the acute phase, which could help the development of new therapies to aid the preclinical tests of drugs for their application in Chagas disease.
Keywords: acute chagas disease; cardiac histopathology; mouse model; pathogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Response to Infection by Trypanosoma cruzi in a Murine Model.Front Vet Sci. 2020 Oct 6;7:568745. doi: 10.3389/fvets.2020.568745. eCollection 2020. Front Vet Sci. 2020. PMID: 33134353 Free PMC article.
-
Altered Cardiomyocyte Function and Trypanosoma cruzi Persistence in Chagas Disease.Am J Trop Med Hyg. 2016 May 4;94(5):1028-33. doi: 10.4269/ajtmh.15-0255. Epub 2016 Mar 14. Am J Trop Med Hyg. 2016. PMID: 26976879 Free PMC article.
-
Physical Exercise Promotes a Reduction in Cardiac Fibrosis in the Chronic Indeterminate Form of Experimental Chagas Disease.Front Immunol. 2021 Nov 4;12:712034. doi: 10.3389/fimmu.2021.712034. eCollection 2021. Front Immunol. 2021. PMID: 34804007 Free PMC article.
-
[Chagasic myocardiopathy: historical perspective].Medicina (B Aires). 1999;59 Suppl 2:25-40. Medicina (B Aires). 1999. PMID: 10668240 Review. Spanish.
-
Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease.Int Arch Allergy Immunol. 1997 Oct;114(2):103-10. doi: 10.1159/000237653. Int Arch Allergy Immunol. 1997. PMID: 9338602 Review.
References
-
- Chagas C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz. 1909;1:159–218. doi: 10.1590/S0074-02761909000200008. - DOI
-
- Organización Panamericana de la Salud . Guía para el Diagnóstico y el Tratamiento de la Enfermedad de Chagas. Washington, D.C. OPS; Philadelphia, PA, USA: 2018.
-
- Rossi M.A., Tanowitz H.B., Malvestio L.M., Celes M.R., Campos E.C., Blefari V., Prado C.M. Coronary Microvascular Disease in Chronic Chagas Cardiomyopathy Including an Overview on History, Pathology, and Other Proposed Pathogenic Mechanisms. PLoS Negl. Trop. Dis. 2010;4:e674. doi: 10.1371/journal.pntd.0000674. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

