In Vitro Antibody-Dependent Enhancement of SARS-CoV-2 Infection Could Be Abolished by Adding Human IgG
- PMID: 37764916
- PMCID: PMC10535176
- DOI: 10.3390/pathogens12091108
In Vitro Antibody-Dependent Enhancement of SARS-CoV-2 Infection Could Be Abolished by Adding Human IgG
Abstract
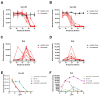
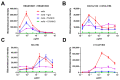
Evidence of antibody-dependent enhancement (ADE) of other viruses has raised concerns about the safety of SARS-CoV-2 vaccines and antibody therapeutics. In vitro studies have shown ADE of SARS-CoV-2 infection. In this study, we also found that vaccination/convalescent sera and some approved monoclonal antibodies can enhance SARS-CoV-2 infection of FcR-expressing B cells in vitro. However, the enhancement of SARS-CoV-2 infection can be prevented by blocking Fc-FcR interaction through the addition of human serum/IgG or the introduction of mutations in the Fc portion of the antibody. It should be noted that ADE activity observed on FcR-expressing cells in vitro may not necessarily reflect the situation in vivo; therefore, animal and clinical data should be included for ADE evaluation.
Keywords: COVID-19; SARS-CoV-2; antibody-dependent enhancement; convalescent serum; vaccination serum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Multiple Routes of Antibody-Dependent Enhancement of SARS-CoV-2 Infection.Microbiol Spectr. 2022 Apr 27;10(2):e0155321. doi: 10.1128/spectrum.01553-21. Epub 2022 Mar 23. Microbiol Spectr. 2022. PMID: 35319248 Free PMC article.
-
Antibody-Dependent Enhancement of SARS-CoV-2 Infection Is Mediated by the IgG Receptors FcγRIIA and FcγRIIIA but Does Not Contribute to Aberrant Cytokine Production by Macrophages.mBio. 2021 Oct 26;12(5):e0198721. doi: 10.1128/mBio.01987-21. Epub 2021 Sep 28. mBio. 2021. PMID: 34579572 Free PMC article.
-
Neutralizing antibodies from the rare convalescent donors elicited antibody-dependent enhancement of SARS-CoV-2 variants infection.Front Med (Lausanne). 2022 Oct 19;9:952697. doi: 10.3389/fmed.2022.952697. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36341247 Free PMC article.
-
SARS-CoV-2 Related Antibody-Dependent Enhancement Phenomena In Vitro and In Vivo.Microorganisms. 2023 Apr 13;11(4):1015. doi: 10.3390/microorganisms11041015. Microorganisms. 2023. PMID: 37110438 Free PMC article. Review.
-
Neutralizing Antibodies and Antibody-Dependent Enhancement in COVID-19: A Perspective.J Indian Inst Sci. 2022;102(2):671-687. doi: 10.1007/s41745-021-00268-8. Epub 2022 Feb 4. J Indian Inst Sci. 2022. PMID: 35136306 Free PMC article. Review.
Cited by
-
Fc gamma receptor polymorphisms in antibody therapy: implications for bioassay development to enhance product quality.Antib Ther. 2025 Jan 21;8(2):87-98. doi: 10.1093/abt/tbaf003. eCollection 2025 Apr. Antib Ther. 2025. PMID: 40177643 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

