Rabbits as Animal Models for Anti-Tick Vaccine Development: A Global Scenario
- PMID: 37764925
- PMCID: PMC10536012
- DOI: 10.3390/pathogens12091117
Rabbits as Animal Models for Anti-Tick Vaccine Development: A Global Scenario
Abstract
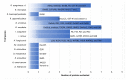
Studies evaluating candidate tick-derived proteins as anti-tick vaccines in natural hosts have been limited due to high costs. To overcome this problem, animal models are used in immunization tests. The aim of this article was to review the use of rabbits as an experimental model for the evaluation of tick-derived proteins as vaccines. A total of 57 tick proteins were tested for their immunogenic potential using rabbits as models for vaccination. The most commonly used rabbit breeds were New Zealand (73.8%), Japanese white (19%), Californians (4.8%) and Flemish lop-eared (2.4%) rabbits. Anti-tick vaccines efficacy resulted in up to 99.9%. Haemaphysalis longicornis (17.9%) and Ornithodoros moubata (12.8%) were the most common tick models in vaccination trials. Experiments with rabbits have revealed that some proteins (CoAQP, OeAQP, OeAQP1, Bm86, GST-Hl, 64TRP, serpins and voraxin) can induce immune responses against various tick species. In addition, in some cases it was possible to determine that the vaccine efficacy in rabbits was similar to that of experiments performed on natural hosts (e.g., Bm86, IrFER2, RmFER2, serpins and serine protease inhibitor). In conclusion, results showed that prior to performing anti-tick vaccination trials using natural hosts, rabbits can be used as suitable experimental models for these studies.
Keywords: antigen; humoral and adaptive response; immunization; rabbit; tick.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Function-guided selection of midgut antigens from Ornithodoros erraticus ticks and an evaluation of their protective efficacy in rabbits.Vet Parasitol. 2019 Aug;272:1-12. doi: 10.1016/j.vetpar.2019.06.016. Epub 2019 Jun 28. Vet Parasitol. 2019. PMID: 31395198
-
Subolesin vaccination inhibits blood feeding and reproduction of Haemaphysalis longicornis in rabbits.Parasit Vectors. 2020 Sep 18;13(1):478. doi: 10.1186/s13071-020-04359-w. Parasit Vectors. 2020. PMID: 32948229 Free PMC article.
-
Evaluation and comparison of the potential of two ferritins as anti-tick vaccines against Haemaphysalis longicornis.Parasit Vectors. 2014 Oct 12;7:482. doi: 10.1186/s13071-014-0482-x. Parasit Vectors. 2014. PMID: 25306467 Free PMC article.
-
Rhipicephalus (Boophilus) microplus embryo proteins as target for tick vaccine.Vet Immunol Immunopathol. 2012 Jul 15;148(1-2):149-56. doi: 10.1016/j.vetimm.2011.05.011. Epub 2011 May 7. Vet Immunol Immunopathol. 2012. PMID: 21620488 Review.
-
Tick-Encoded serine proteinase inhibitors (serpins); potential target antigens for tick vaccine development.J Vet Med Sci. 2001 Oct;63(10):1063-9. doi: 10.1292/jvms.63.1063. J Vet Med Sci. 2001. PMID: 11714020 Review.
Cited by
-
Cross-species immunoprotective antigens (subolesin, ferritin 2 and P0) provide protection against Rhipicephalus sanguineus sensu lato.Parasit Vectors. 2024 Jan 3;17(1):3. doi: 10.1186/s13071-023-06079-3. Parasit Vectors. 2024. PMID: 38172894 Free PMC article.
-
Immunoprotection Provided by Salivary and Intestinal Protein-Based Antigens Against the Ixodid Tick Amblyomma sculptum.Vaccines (Basel). 2025 Jan 28;13(2):136. doi: 10.3390/vaccines13020136. Vaccines (Basel). 2025. PMID: 40006683 Free PMC article.
-
Fourteen anti-tick vaccine targets are variably conserved in cattle fever ticks.Parasit Vectors. 2025 Apr 15;18(1):140. doi: 10.1186/s13071-025-06683-5. Parasit Vectors. 2025. PMID: 40234925 Free PMC article.
-
In vitro identification of neutralizing epitopes of Rhipicephalus microplus serpin 17 (RmS-17).Vaccine. 2024 Aug 13;42(20):126161. doi: 10.1016/j.vaccine.2024.126161. Epub 2024 Jul 26. Vaccine. 2024. PMID: 39060200 Free PMC article.
References
-
- Brisola C. Medical and Veterinary Entomology. Publishing Athens; Athens, Greece: 2011. Mites (ticks and others) pp. 263–315.
-
- Alcantara E., Ferreira da Silva C., Ávila R., Pacheco R., Muñoz L., Honorio D. Ticks (Acari: Argasidae and Ixodidae) infesting amphibians and reptiles in northeastern Brazil. Syst. Appl. Acarol. 2018;23:1497. doi: 10.11158/saa.23.8.1. - DOI
-
- Santos M., Bahiense T., Silva A., Onofrio V., Barral T., Souza B., Lira-da-Silva R., Biondi I., Meyer R., Portela R. Ticks and associated pathogens from rescued wild animals in rainforest fragments of northeastern Brazil. Front. Vet. Sci. 2020;7:177. doi: 10.3389/fvets.2020.00177. - DOI - PMC - PubMed
-
- Cortés-Vecino J. Changes in the distribution and abundance of ticks and their relationship with global warming. J. Vet. Med. Zoot. 2010;57:65–75.
Publication types
Grants and funding
- 88881.068421/2014-01/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- 465678/2014-9/National Council for Scientific and Technological Development
- 21/2551-0002221-3/Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul
- E-26/210.012/2018/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
- SEI-260003/001743/2023/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
LinkOut - more resources
Full Text Sources
Research Materials

