A Multilayered Imaging and Microfluidics Approach for Evaluating the Effect of Fibrinolysis in Staphylococcus aureus Biofilm Formation
- PMID: 37764949
- PMCID: PMC10534389
- DOI: 10.3390/pathogens12091141
A Multilayered Imaging and Microfluidics Approach for Evaluating the Effect of Fibrinolysis in Staphylococcus aureus Biofilm Formation
Abstract
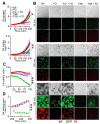
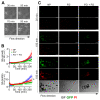
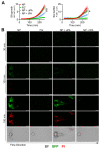
The recognition of microbe and extracellular matrix (ECM) is a recurring theme in the humoral innate immune system. Fluid-phase molecules of innate immunity share regulatory roles in ECM. On the other hand, ECM elements have immunological functions. Innate immunity is evolutionary and functionally connected to hemostasis. Staphylococcus aureus (S. aureus) is a major cause of hospital-associated bloodstream infections and the most common cause of several life-threatening conditions such as endocarditis and sepsis through its ability to manipulate hemostasis. Biofilm-related infection and sepsis represent a medical need due to the lack of treatments and the high resistance to antibiotics. We designed a method combining imaging and microfluidics to dissect the role of elements of the ECM and hemostasis in triggering S. aureus biofilm by highlighting an essential role of fibrinogen (FG) in adhesion and formation. Furthermore, we ascertained an important role of the fluid-phase activation of fibrinolysis in inhibiting biofilm of S. aureus and facilitating an antibody-mediated response aimed at pathogen killing. The results define FG as an essential element of hemostasis in the S. aureus biofilm formation and a role of fibrinolysis in its inhibition, while promoting an antibody-mediated response. Understanding host molecular mechanisms influencing biofilm formation and degradation is instrumental for the development of new combined therapeutic approaches to prevent the risk of S. aureus biofilm-associated diseases.
Keywords: Staphylococcus aureus; biofilm; extracellular matrix; fibrinolysis; hemostasis; innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Koyama K., Madoiwa S., Nunomiya S., Koinuma T., Wada M., Sakata A., Ohmori T., Mimuro J., Sakata Y. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: A prospective observational study. Crit. Care. 2014;18:R13. doi: 10.1186/cc13190. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

