Characterization of Bovine Intraepithelial T Lymphocytes in the Gut
- PMID: 37764981
- PMCID: PMC10535955
- DOI: 10.3390/pathogens12091173
Characterization of Bovine Intraepithelial T Lymphocytes in the Gut
Abstract
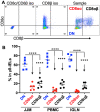
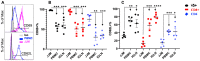
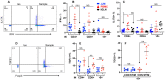
Intraepithelial T lymphocytes (T-IELs), which constitute over 50% of the total T lymphocytes in the animal, patrol the mucosal epithelial lining to defend against pathogen invasion while maintaining gut homeostasis. In addition to expressing T cell markers such as CD4 and CD8, T-IELs display T cell receptors (TCR), including either TCRαβ or TCRγδ. Both humans and mice share similar T-IEL subsets: TCRγδ+, TCRαβ+CD8αα+, TCRαβ+CD4+, and TCRαβ+CD8αβ+. Among these subsets, human T-IELs are predominantly TCRαβ+ (over 80%), whereas those in mice are mostly TCRγδ+ (~60%). Of note, the majority of the TCRγδ+ subset expresses CD8αα in both species. Although T-IELs have been extensively studied in humans and mice, their profiles in cattle have not been well examined. Our study is the first to characterize bovine T-IELs using flow cytometry, where we identified several distinct features. The percentage of TCRγδ+ was comparable to that of TCRαβ+ T-IELs (both ~50% of CD3+), and the majority of bovine TCRγδ+ T-IELs did not express CD8 (CD8-) (above 60%). Furthermore, about 20% of TCRαβ+ T-IELs were CD4+CD8αβ+, and the remaining TCRαβ+ T-IELs were evenly distributed between CD4+ and CD8αβ+ (~40% of TCRαβ+ T-IELs each) with no TCRαβ+CD8αα+ identified. Despite these unique properties, bovine T-IELs, similar to those in humans and mice, expressed a high level of CD69, an activation and tissue-retention marker, and a low level of CD62L, a lymphoid adhesion marker. Moreover, bovine T-IELs produced low levels of inflammatory cytokines such as IFNγ and IL17A, and secreted small amounts of the immune regulatory cytokine TGFβ1. Hence, bovine T-IELs' composition largely differs from that of human and mouse, with the dominance of the CD8- population among TCRγδ+ T-IELs, the substantial presence of TCRαβ+CD4+CD8αβ+ cells, and the absence of TCRαβ+CD8αα+ T-IELs. These results provide the groundwork for conducting future studies to examine how bovine T-IELs respond to intestinal pathogens and maintain the integrity of the gut epithelial barrier in animals.
Keywords: CD4; CD8; IFNγ; IL17A; T-IELs; TCRαβ; TCRγδ; TGFβ1; cattle; intraepithelial T lymphocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Edelblum K.L., Singh G., Odenwald M.A., Lingaraju A., El Bissati K., Mcleod R., Sperling A.I., Turner J.R. γδ intraepithelial lymphocyte migration limits transepithelial pathogen invasion and systemic disease in mice. Gastroenterology. 2015;148:1417–1426. doi: 10.1053/j.gastro.2015.02.053. - DOI - PMC - PubMed
-
- Ismail A.S., Severson K.M., Vaishnava S., Behrendt C.L., Yu X., Benjamin J.L., Ruhn K.A., Hou B., Defranco A.L., Yarovinsky F., et al. γδ intraepithelial lymphocytes are essential mediators of host–microbial homeostasis at the intestinal mucosal surface. Proc. Natl. Acad. Sci. USA. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

