Case Series of Primaquine-Induced Haemolytic Events in Controlled Trials with G6PD Screening
- PMID: 37764985
- PMCID: PMC10537757
- DOI: 10.3390/pathogens12091176
Case Series of Primaquine-Induced Haemolytic Events in Controlled Trials with G6PD Screening
Abstract
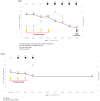
Primaquine for radical cure of Plasmodium vivax malaria poses a potentially life-threatening risk of haemolysis in G6PD-deficient patients. Herein, we review five events of acute haemolytic anaemia following the administration of primaquine in four malaria trials from Indonesia, the Solomon Islands, and Vietnam. Five males aged 9 to 48 years were improperly classified as G6PD-normal by various screening procedures and included as subjects in trials of anti-relapse therapy with daily primaquine. Routine safety monitoring by physical examination, urine inspection, and blood haemoglobin (Hb) assessment were performed in all those trials. Early signs of acute haemolysis, i.e., dark urine and haemoglobin drop >20%, occurred only after day 3 and as late as day 8 of primaquine dosing. All patients were hospitalized and fully recovered, all but one following blood transfusion rescue. Hb nadir was 4.7 to 7.9 g/dL. Hospitalization was for 1 to 7 days. Hb levels returned to baseline values 3 to 10 days after transfusion. Failed G6PD screening procedures in these trials led G6PD-deficient patients to suffer harmful exposures to primaquine. The safe application of primaquine anti-relapse therapy requires G6PD screening and anticipation of its failure with a means of prompt detection and rescue from the typically abrupt haemolytic crisis.
Keywords: G6PD deficiency; G6PD screening; acute haemolytic anaemia; primaquine; randomized controlled trials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization . World Malaria Report 2022. World Health Organization; Geneva, Switzerland: 2022.
-
- World Health Organization . WHO Guidelines for Malaria, 3 June 2022. World Health Organization; Geneva, Switzerland: 2022.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

