The Development of Magnolol-Loaded Intravenous Emulsion with Low Hepatotoxic Potential
- PMID: 37765070
- PMCID: PMC10537714
- DOI: 10.3390/ph16091262
The Development of Magnolol-Loaded Intravenous Emulsion with Low Hepatotoxic Potential
Abstract
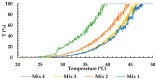
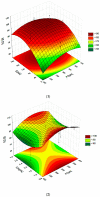
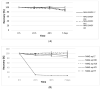
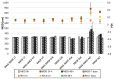
Intestinal failure-associated liver disease (IFALD) is a severe liver injury occurring due to factors related to intestinal failure and parenteral nutrition administration. Different approaches are studied to reduce the risk or ameliorate the course of IFALD, including providing omega-3 fatty acids instead of soybean oil-based lipid emulsion or administering active compounds that exert a hepatoprotective effect. This study aimed to develop, optimize, and characterize magnolol-loaded intravenous lipid emulsion for parenteral nutrition. The preformulation studies allowed for chosen oils mixture of the highest capacity of magnolol solubilization. Then, magnolol-loaded SMOFlipid was developed using the passive incorporation method. The Box-Behnken design and response surface methodology were used to optimize the entrapment efficiency. The optimal formulation was subjected to short-term stress tests, and its effect on normal human liver cells and erythrocytes was determined using the MTT and hemolysis tests, respectively. The optimized magnolol-loaded SMOFlipid was characterized by the mean droplet diameter of 327.6 ± 2.9 nm with a polydispersity index of 0.12 ± 0.02 and zeta potential of -32.8 ± 1.2 mV. The entrapment efficiency of magnolol was above 98%, and pH and osmolality were sufficient for intravenous administration. The magnolol-loaded SMOFlipid samples showed a significantly lower toxic effect than bare SMOFlipid in the same concentration on THLE-2 cells, and revealed an acceptable hemolytic effect of 8.3%. The developed formulation was characterized by satisfactory stability. The in vitro studies showed the reduced cytotoxic effect of MAG-SMOF applied in high concentrations compared to bare SMOFlipid and the non-hemolytic effect on human blood cells. The magnolol-loaded SMOFlipid is promising for further development of hepatoprotective lipid emulsion for parenteral nutrition.
Keywords: Box–Behnken design; liver disease; magnolol; parenteral nutrition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lauriti G., Zani A., Aufieri R., Cananzi M., Chiesa P.L., Eaton S., Pierro A. Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: A systematic review. J. Parenter. Enteral Nutr. 2014;38:70–85. doi: 10.1177/0148607113496280. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

