Anxiolytic- like Effects by trans-Ferulic Acid Possibly Occur through GABAergic Interaction Pathways
- PMID: 37765079
- PMCID: PMC10535412
- DOI: 10.3390/ph16091271
Anxiolytic- like Effects by trans-Ferulic Acid Possibly Occur through GABAergic Interaction Pathways
Abstract
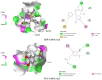
Numerous previous studies reported that ferulic acid exerts anxiolytic activity. However, the mechanisms have yet to be elucidated. The current study aimed to investigate the anxiolytic effect of trans-ferulic acid (TFA), a stereoisomer of ferulic acid, and evaluated its underlying mechanism using in vivo and computational studies. For this, different experimental doses of TFA (25, 50, and 75 mg/kg) were administered orally to Swiss albino mice, and various behavioral methods of open field, hole board, swing box, and light-dark tests were carried out. Diazepam (DZP), a positive allosteric modulator of the GABAA receptor, was employed as a positive control at a dose of 2 mg/kg, and distilled water served as a vehicle. Additionally, molecular docking was performed to estimate the binding affinities of the TFA and DZP toward the GABAA receptor subunits of α2 and α3, which are associated with the anxiolytic effect; visualizations of the ligand-receptor interaction were carried out using various computational tools. Our findings indicate that TFA dose-dependently reduces the locomotor activity of the animals in comparison with the controls, calming their behaviors. In addition, TFA exerted the highest binding affinity (-5.8 kcal/mol) to the α2 subunit of the GABAA receptor by forming several hydrogen and hydrophobic bonds. Taken together, our findings suggest that TFA exerts a similar effect to DZP, and the compound exerts moderate anxiolytic activity through the GABAergic interaction pathway. We suggest further clinical studies to develop TFA as a reliable anxiolytic agent.
Keywords: GABAergic system; anxiolytic effect; in silico; molecular docking; trans-ferulic acid.
Conflict of interest statement
The authors state that they have no conflict of interest to disclose.
Figures





References
-
- Rende R., Waldman I.J. Behavioral and molecular genetics and developmental psychopathology. Dev. Psychopathol. 2015;10:427–464.
LinkOut - more resources
Full Text Sources

