Neuroprotective Effect of Nosustrophine in a 3xTg Mouse Model of Alzheimer's Disease
- PMID: 37765114
- PMCID: PMC10535028
- DOI: 10.3390/ph16091306
Neuroprotective Effect of Nosustrophine in a 3xTg Mouse Model of Alzheimer's Disease
Abstract
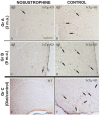
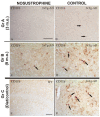
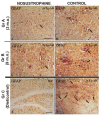
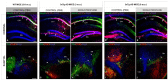
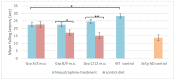
Neurodegeneration, characterized by the progressive deterioration of neurons and glial cells, is a feature of Alzheimer's disease (AD). The present study aims to demonstrate that the onset and early progression of neurodegenerative processes in transgenic mice models of AD can be delayed by a cocktail of neurotrophic factors and derived peptides named Nosustrophine, a nootropic supplement made by a peptide complex extracted from the young porcine brain, ensuring neuroprotection and improving neuro-functional recovery. Experimental 3xTg-APP/Bin1/COPS5 transgenic mice models of AD were treated with Nosustrophine at two different early ages, and their neuropathological hallmark and behavior response were analyzed. Results showed that Nosustrophine increased the activity of the immune system and reduced pathological changes in the hippocampus and cortex by halting the development of amyloid plaques, mainly seen in mice of 3-4 months of age, indicating that its effect is more preventive than therapeutic. Taken together, the results indicate the potent neuroprotective activity of Nosustrophine and its stimulating effects on neuronal plasticity. This study shows for the first time an effective therapy using nootropic supplements against degenerative diseases, although further investigation is needed to understand their molecular pathways.
Keywords: 3xTg-AD; Alzheimer’s disease; neuroprotection; nosustrophine; porcine brain extract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Nosustrophine: An Epinutraceutical Bioproduct with Effects on DNA Methylation, Histone Acetylation and Sirtuin Expression in Alzheimer's Disease.Pharmaceutics. 2022 Nov 12;14(11):2447. doi: 10.3390/pharmaceutics14112447. Pharmaceutics. 2022. PMID: 36432638 Free PMC article.
-
SLOH, a carbazole-based fluorophore, mitigates neuropathology and behavioral impairment in the triple-transgenic mouse model of Alzheimer's disease.Neuropharmacology. 2018 Mar 15;131:351-363. doi: 10.1016/j.neuropharm.2018.01.003. Epub 2018 Jan 5. Neuropharmacology. 2018. PMID: 29309769
-
Retinal macroglia changes in a triple transgenic mouse model of Alzheimer's disease.Exp Eye Res. 2014 Oct;127:252-60. doi: 10.1016/j.exer.2014.08.006. Epub 2014 Aug 19. Exp Eye Res. 2014. PMID: 25149907 Free PMC article.
-
Mechanisms of Hydrogen Sulfide against the Progression of Severe Alzheimer's Disease in Transgenic Mice at Different Ages.Pharmacology. 2019;103(1-2):50-60. doi: 10.1159/000494113. Epub 2018 Nov 16. Pharmacology. 2019. PMID: 30448835
-
Neuroprotective Effect of SLM, a Novel Carbazole-Based Fluorophore, on SH-SY5Y Cell Model and 3xTg-AD Mouse Model of Alzheimer's Disease.ACS Chem Neurosci. 2017 Mar 15;8(3):676-685. doi: 10.1021/acschemneuro.6b00388. Epub 2016 Dec 29. ACS Chem Neurosci. 2017. PMID: 28032988
Cited by
-
Preventive Role of Cocoa-Enriched Extract Against Neuroinflammation in Mice.Neurol Int. 2025 Mar 24;17(4):47. doi: 10.3390/neurolint17040047. Neurol Int. 2025. PMID: 40278418 Free PMC article.
-
Therapeutic Options in Alzheimer's Disease: From Classic Acetylcholinesterase Inhibitors to Multi-Target Drugs with Pleiotropic Activity.Life (Basel). 2024 Nov 26;14(12):1555. doi: 10.3390/life14121555. Life (Basel). 2024. PMID: 39768263 Free PMC article.
References
-
- Zhang L., Fang Y., Lian Y., Chen Y., Wu T., Zheng Y., Zong H., Sun L., Zhang R., Wang Z., et al. Brain-Derived Neurotrophic Factor Ameliorates Learning Deficits in a Rat Model of Alzheimer’s Disease Induced by Aβ1-42. PLoS ONE. 2015;10:e0122415. doi: 10.1371/journal.pone.0122415. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical

