Topical Micro-Emulsion of 5-Fluorouracil by a Twin Screw Processor-Based Novel Continuous Manufacturing Process for the Treatment of Skin Cancer: Preparation and In Vitro and In Vivo Evaluations
- PMID: 37765146
- PMCID: PMC10534867
- DOI: 10.3390/pharmaceutics15092175
Topical Micro-Emulsion of 5-Fluorouracil by a Twin Screw Processor-Based Novel Continuous Manufacturing Process for the Treatment of Skin Cancer: Preparation and In Vitro and In Vivo Evaluations
Abstract
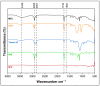
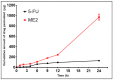
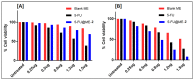
5-Fluorouracil (5-FU), a BCS class III drug, has low oral bioavailability and is cytotoxic in nature causing severe systemic side effects when administered through the intravenous route. Topical drug delivery could potentially mitigate the systemic side-effects. Microemulsions (MEs) would be an apt solution due to enhanced partitioning of the drug to the skin. However, conventional methods for preparing MEs are inefficient since they are not continuous and are very tedious and time-consuming processes hence revealing the need for the development of continuous manufacturing technology. In our study, 5-FU MEs were prepared using a continuous manufacturing Twin Screw Process (TSP) and its efficiency in the treatment of skin cancer was evaluated. Water-in-oil MEs were prepared using isopropyl myristate as the oil phase and Aerosol OT and Tween 80 as the surfactants. The average particle size was observed to be 178 nm. Transmission electron microscopy was employed to confirm the size and shape of the MEs. FTIR study proved no physical or chemical interaction between the excipients and the drug. In vitro drug release using vertical diffusion cells and ex vivo skin permeation studies showed that the drug was released sustainably and permeated across the skin, respectively. In in vitro cytotoxicity studies, 5-FU MEs were accessed in HaCat and A431 cell lines to determine percentage cell viability and IC50. Skin irritation and histopathological examination implied that the 5-FU MEs did not cause any significant irritation to the skin. In vivo pharmacodynamics studies in rats suggested that the optimised formulation was effective in treating squamous cell carcinoma (SCC). Therefore, 5-FU MEs efficiently overcame the various drawbacks faced during oral and intravenous drug delivery. Also, TSP proved to be a technique that overcomes the various problems associated with the conventional methods of preparing MEs.
Keywords: 5-Fluorouracil; continuous manufacturing; microemulsion; skin cancer; squamous cell carcinoma (SCC); twin-screw processor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Heistein J.B., Acharya U. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Malignant Melanoma. - PubMed
-
- Orthaber K., Pristovnik M., Skok K., Perić B., Maver U. Skin Cancer and Its Treatment: Novel Treatment Approaches with Emphasis on Nanotechnology. J. Nanomater. 2017;2017:2606271. doi: 10.1155/2017/2606271. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

