Molecular Hybridization as a Strategy for Developing Artemisinin-Derived Anticancer Candidates
- PMID: 37765156
- PMCID: PMC10536797
- DOI: 10.3390/pharmaceutics15092185
Molecular Hybridization as a Strategy for Developing Artemisinin-Derived Anticancer Candidates
Abstract
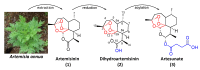
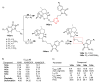
Artemisinin is a natural compound extracted from Artemisia species belonging to the Asteraceae family. Currently, artemisinin and its derivatives are considered among the most significant small-molecule antimalarial drugs. Artemisinin and its derivatives have also been shown to possess selective anticancer properties, however, there are several limitations and gaps in knowledge that retard their repurposing as effective anticancer agents. Hybridization resulting from a covalent combination of artemisinin with one or more active pharmacophores has emerged as a promising approach to overcome several issues. The variety of hybridization partners allows improvement in artemisinin activity by tuning the ability of conjugated artemisinin to interact with various molecule targets involved in multiple biological pathways. This review highlights the current scenario of artemisinin-derived hybrids with potential anticancer activity. The synthetic approaches to achieve the corresponding hybrids and the structure-activity relationships are discussed to facilitate further rational design of more effective candidates.
Keywords: anticancer activity; artemisinin; artesunate; click chemistry; dihydroartemisinin; hybrids; molecular hybridization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
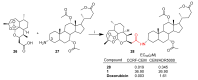


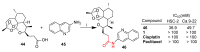

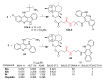








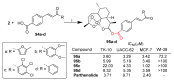







References
Publication types
LinkOut - more resources
Full Text Sources

