Meropenem PK/PD Variability and Renal Function: "We Go Together"
- PMID: 37765207
- PMCID: PMC10534409
- DOI: 10.3390/pharmaceutics15092238
Meropenem PK/PD Variability and Renal Function: "We Go Together"
Abstract
Background: Meropenem is a carbapenem antibiotic widely employed for serious bacterial infections. Therapeutic drug monitoring (TDM) is a strategy to optimize dosing, especially in critically ill patients. This study aims to show how TDM influences the management of meropenem in a real-life setting, not limited to intensive care units.
Methods: From December 2021 to February 2022, we retrospectively analyzed 195 meropenem serum concentrations (Css). We characterized patients according to meropenem exposure, focusing on the renal function impact.
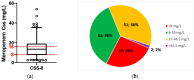
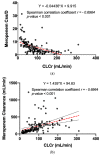
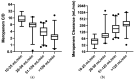
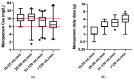
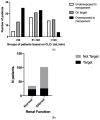
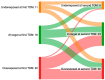
Results: A total of 36% (n = 51) of the overall observed patients (n = 144) were in the therapeutic range (8-16 mg/L), whereas 64% (n = 93) required a meropenem dose modification (37 patients (26%) underexposed; 53 (38%) overexposed). We found a strong relationship between renal function and meropenem concentrations (correlation coefficient = -0.7; p-value < 0.001). We observed different dose-normalized meropenem exposure (Css/D) among renal-impaired (severe and moderate), normal, and hyperfiltrating patients, with a median (interquartile range) of 13.1 (10.9-20.2), 7.9 (6.1-9.5), 3.8 (2.6-6.0), and 2.4 (1.6-2.7), respectively (p-value < 0.001).
Conclusions: Meropenem TDM in clinical practice allows modification of dosing in patients inadequately exposed to meropenem to maximize antibiotic efficacy and minimize the risk of antibiotic resistance, especially in renal alterations despite standard dose adaptations.
Keywords: antibiotics; antimicrobial resistance; clinical pharmacology; continuous infusion; critical illness; dose optimization; meropenem; pharmacokinetic; renal function; therapeutic drug monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Roberts J.A., Abdul-Aziz M.-H., Lipman J., Mouton J.W., Vinks A.A., Felton T.W., Hope W.W., Farkas A., Neely M.N., Schentag J.J., et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014;14:498–509. doi: 10.1016/S1473-3099(14)70036-2. - DOI - PMC - PubMed
-
- Taccone F.S., Laterre P.-F., Dugernier T., Spapen H., Delattre I., Witebolle X., De Backer D., Layeux B., Wallemacq P., Vincent J.-L., et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care. 2010;14:R126. doi: 10.1186/cc9091. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

