Biomimetic Electrospun Self-Assembling Peptide Scaffolds for Neural Stem Cell Transplantation in Neural Tissue Engineering
- PMID: 37765230
- PMCID: PMC10536048
- DOI: 10.3390/pharmaceutics15092261
Biomimetic Electrospun Self-Assembling Peptide Scaffolds for Neural Stem Cell Transplantation in Neural Tissue Engineering
Abstract
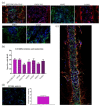
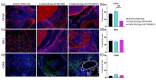
Spinal cord regeneration using stem cell transplantation is a promising strategy for regenerative therapy. Stem cells transplanted onto scaffolds that can mimic natural extracellular matrix (ECM) have the potential to significantly improve outcomes. In this study, we strived to develop a cell carrier by culturing neural stem cells (NSCs) onto electrospun 2D and 3D constructs made up of specific crosslinked functionalized self-assembling peptides (SAPs) featuring enhanced biomimetic and biomechanical properties. Morphology, architecture, and secondary structures of electrospun scaffolds in the solid-state and electrospinning solution were studied step by step. Morphological studies showed the benefit of mixed peptides and surfactants as additives to form thinner, uniform, and defect-free fibers. It has been observed that β-sheet conformation as evidence of self-assembling has been predominant throughout the process except for the electrospinning solution. In vitro NSCs seeded on electrospun SAP scaffolds in 2D and 3D conditions displayed desirable proliferation, viability, and differentiation in comparison to the gold standard. In vivo biocompatibility assay confirmed the permissibility of implanted fibrous channels by foreign body reaction. The results of this study demonstrated that fibrous 2D/3D electrospun SAP scaffolds, when shaped as micro-channels, can be suitable to support NSC transplantation for regeneration following spinal cord injury.
Keywords: 2D/3D scaffolds; electrospinning; regenerative medicine; secondary structures; self-assembling peptides; spinal cord injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Kusindarta D.L., Wihadmadyatami H. Tissue Regeneration. IntechOpen; London, UK: 2018. The role of extracellular matrix in tissue regeneration. - DOI
-
- Onuwaje I., Phillips J.B. Handbook of Innovations in Central Nervous System Regenerative Medicine. Elsevier; Amsterdam, The Netherlands: 2020. Three-dimensional culture systems in central nervous system research; pp. 571–601.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

