A Species-Specific Anti-Human P2X7 Monoclonal Antibody Reduces Graft-versus-Host Disease in Humanised Mice
- PMID: 37765233
- PMCID: PMC10536354
- DOI: 10.3390/pharmaceutics15092263
A Species-Specific Anti-Human P2X7 Monoclonal Antibody Reduces Graft-versus-Host Disease in Humanised Mice
Abstract
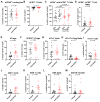
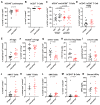
Graft-versus-host disease (GVHD) is a T cell-mediated inflammatory disorder that arises from allogeneic haematopoietic stem cell transplantation and is often fatal. The P2X7 receptor is an extracellular adenosine 5'-triphosphate-gated cation channel expressed on immune cells. Blockade of this receptor with small molecule inhibitors impairs GVHD in a humanised mouse model. A species-specific blocking monoclonal antibody (mAb) (clone L4) for human P2X7 is available, affording the opportunity to determine whether donor (human) P2X7 contributes to the development of GVHD in humanised mice. Using flow cytometric assays of human RPMI 8266 and murine J774 cells, this study confirmed that this mAb bound and impaired human P2X7. Furthermore, this mAb prevented the loss of human regulatory T cells (hTregs) and natural killer (hNK) T cells in vitro. NOD-scid IL2Rγnull mice were injected with 10 × 106 human peripheral blood mononuclear cells (Day 0) and an anti-hP2X7 or control mAb (100 μg i.p. per mouse, Days 0, 2, 4, 6, and 8). The anti-hP2X7 mAb increased hTregs and hNK cells at Day 21. Moreover, anti-hP2X7 mAb-treatment reduced clinical and histological GVHD in the liver and lung compared to the control treatment at disease endpoint. hTregs, hNK, and hNK T cell proportions were increased, and human T helper 17 cell proportions were decreased at endpoint. These studies indicate that blockade of human (donor) P2X7 reduces GVHD development in humanised mice, providing the first direct evidence of a role for donor P2X7 in GVHD.
Keywords: P2RX7; P2X7; T helper 17 cells; biologic; natural killer T cells; natural killer cells; purinergic signalling; regulatory T cells; therapeutic antibody; xenogeneic graft-versus-host disease.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







References
-
- Holtan S.G., Yu J., Choe H.K., Paranagama D., Tang J., Naim A., Galvin J., Joachim Deeg H. Disease progression, treatments, hospitalization, and clinical outcomes in acute GVHD: A multicenter chart review. Bone Marrow Transplant. 2022;57:1581–1585. doi: 10.1038/s41409-022-01764-w. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

