Twin Screw Melt Granulation: A Single Step Approach for Developing Self-Emulsifying Drug Delivery System for Lipophilic Drugs
- PMID: 37765237
- PMCID: PMC10534719
- DOI: 10.3390/pharmaceutics15092267
Twin Screw Melt Granulation: A Single Step Approach for Developing Self-Emulsifying Drug Delivery System for Lipophilic Drugs
Abstract
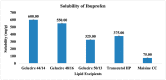
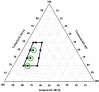
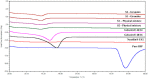
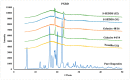
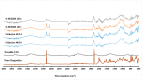
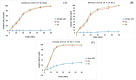
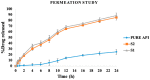
The current research aims to improve the solubility of the poorly soluble drug, i.e., ibuprofen, by developing self-emulsifying drug delivery systems (SEDDS) utilizing a twin screw melt granulation (TSMG) approach. Gelucire® 44/14, Gelucire® 48/16, and Transcutol® HP were screened as suitable excipients for developing the SEDDS formulations. Initially, liquid SEDDS (L-SEDDS) were developed with oil concentrations between 20-50% w/w and surfactant to co-surfactant ratios of 2:1, 4:1, 6:1. The stable formulations of L-SEDDS were transformed into solid SEDDS (S-SEDDS) using a suitable adsorbent carrier and compressed into tablets (T-SEDDS). The S-SEDDS has improved flow, drug release profiles, and permeability compared to pure drugs. The existence of the drug in an amorphous state was confirmed by differential scanning calorimetry (DSC) and powder X-ray diffraction analysis (PXRD). The formulations with 20% w/w and 30% w/w of oil concentration and a 4:1 ratio of surfactant to co-surfactant have resulted in a stable homogeneous emulsion with a globule size of 14.67 ± 0.23 nm and 18.54 ± 0.55 nm. The compressed tablets were found stable after six months of storage at accelerated and long-term conditions. This shows the suitability of the TSMG approach as a single-step continuous manufacturing process for developing S-SEDDS formulations.
Keywords: globule size; liquid SEDDS; polydispersity index; self-emulsifying drug delivery system; solid SEDDS; twin screw melt granulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Twin Screw Melt Granulation: Alternative Approach for Improving Solubility and Permeability of a Non-steroidal Anti-inflammatory Drug Ibuprofen.AAPS PharmSciTech. 2023 Jan 26;24(1):47. doi: 10.1208/s12249-023-02512-z. AAPS PharmSciTech. 2023. PMID: 36703024
-
Formulation and processing of solid self-emulsifying drug delivery systems (HME S-SEDDS): A single-step manufacturing process via hot-melt extrusion technology through response surface methodology.Int J Pharm. 2023 Jun 25;641:123055. doi: 10.1016/j.ijpharm.2023.123055. Epub 2023 May 18. Int J Pharm. 2023. PMID: 37207857 Free PMC article.
-
Development and Characterization of Solid Self-emulsifying Drug Delivery System of Cilnidipine.Chem Pharm Bull (Tokyo). 2015;63(6):408-17. doi: 10.1248/cpb.c14-00326. Chem Pharm Bull (Tokyo). 2015. PMID: 26027464
-
Self-emulsifying Drug Delivery System Improve Oral Bioavailability: Role of Excipients and Physico-chemical Characterization.Pharm Nanotechnol. 2020;8(4):290-301. doi: 10.2174/2211738508666200811104240. Pharm Nanotechnol. 2020. PMID: 32781978 Review.
-
Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs.Biomed Pharmacother. 2004 Apr;58(3):173-82. doi: 10.1016/j.biopha.2004.02.001. Biomed Pharmacother. 2004. PMID: 15082340 Review.
Cited by
-
An Advanced Twin-Screw Granulation Technology: The use of Non-Volatile Solvents with High Solubilizing Capacity.AAPS PharmSciTech. 2024 Jul 31;25(6):174. doi: 10.1208/s12249-024-02890-y. AAPS PharmSciTech. 2024. PMID: 39085532
-
Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation-Where Are We Now?AAPS PharmSciTech. 2024 Feb 14;25(2):37. doi: 10.1208/s12249-024-02749-2. AAPS PharmSciTech. 2024. PMID: 38355916 Review.
-
Sodium Starch Glycolate (SSG) from Sago Starch (Metroxylon sago) as a Superdisintegrant: Synthesis and Characterization.Molecules. 2023 Dec 26;29(1):151. doi: 10.3390/molecules29010151. Molecules. 2023. PMID: 38202734 Free PMC article. Review.
-
Formulation Development of Solid Self-Nanoemulsifying Drug Delivery Systems of Quetiapine Fumarate via Hot-Melt Extrusion Technology: Optimization Using Central Composite Design.Pharmaceutics. 2024 Feb 26;16(3):324. doi: 10.3390/pharmaceutics16030324. Pharmaceutics. 2024. PMID: 38543219 Free PMC article.
-
Self-Emulsifying Drug Delivery Systems (SEDDS): Transition from Liquid to Solid-A Comprehensive Review of Formulation, Characterization, Applications, and Future Trends.Pharmaceutics. 2025 Jan 5;17(1):63. doi: 10.3390/pharmaceutics17010063. Pharmaceutics. 2025. PMID: 39861711 Free PMC article. Review.
References
-
- Kumar S., Dilbaghi N., Saharan R., Bhanjana G. Nanotechnology as Emerging Tool for Enhancing Solubility of Poorly Water-Soluble Drugs. Bionanoscience. 2012;2:227–250. doi: 10.1007/s12668-012-0060-7. - DOI
-
- Sohail Arshad M., Zafar S., Yousef B., Alyassin Y., Ali R., AlAsiri A., Chang M.W., Ahmad Z., Ali Elkordy A., Faheem A., et al. A Review of Emerging Technologies Enabling Improved Solid Oral Dosage Form Manufacturing and Processing. Adv. Drug Deliv. Rev. 2021;178:113840. doi: 10.1016/j.addr.2021.113840. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

