Improved Pharmacokinetic Feasibilities of Mirabegron-1,2-Ethanedisulfonic Acid, Mirabegron-1,5-Naphthalenedisulfonic Acid, and Mirabegron-L-Pyroglutamic Acid as Co-Amorphous Dispersions in Rats and Mice
- PMID: 37765246
- PMCID: PMC10536516
- DOI: 10.3390/pharmaceutics15092277
Improved Pharmacokinetic Feasibilities of Mirabegron-1,2-Ethanedisulfonic Acid, Mirabegron-1,5-Naphthalenedisulfonic Acid, and Mirabegron-L-Pyroglutamic Acid as Co-Amorphous Dispersions in Rats and Mice
Abstract
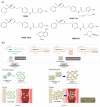
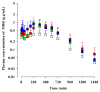
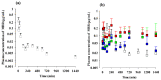
Mirabegron (MBR) is a β3-adrenoceptor agonist used for treating overactive bladder syndrome. Due to its poor solubility and low bioavailability (F), the development of novel MBR formulations has garnered increasing attention. Recently, co-amorphous dispersions of MBR, such as MBR-1,2-ethanedisulfonic acid (MBR-EFA), MBR-1,5-naphthalenedisulfonic acid (MBR-NDA), and MBR-L-pyroglutamic acid (MBR-PG), have been developed, showing improved solubility and thermodynamic stability. Nevertheless, the pharmacokinetic feasibility of these co-amorphous dispersions has not been evaluated. Therefore, this study aimed to characterize the pharmacokinetic profiles of MBR-EFA, MBR-NDA, and MBR-PG in rats and mice. Our results exhibited that relative F24h and AUC0-24h values of MBR in MBR-EFA, MBR-NDA, and MBR-PG rats were increased by 143-195% compared with the MBR rats. The absolute F24h, relative F24h, and AUC0-24h values of MBR in MBR-EFA and MBR-NDA mice were enhanced by 178-234% compared with the MBR mice. In tissue distribution, MBR was extensively distributed in the gastrointestinal tract, liver, kidneys, lung, and heart of mice. Notably, MBR distribution in the liver, kidneys, and lung was considerably high in MBR-EFA, MBR-NDA, or MBR-PG mice compared with MBR mice. These findings highlight the potential of these co-amorphous dispersions to enhance oral F of MBR.
Keywords: bioavailability; co-amorphous dispersion; mirabegron; pharmacokinetics; tissue distribution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chen H.L., Chen T.C., Chang H.M., Juan Y.S., Huang W.H., Pan H.F., Chang Y.C., Wu C.M., Wang Y.L., Lee H.Y. Mirabegron is alternative to antimuscarinic agents for overactive bladder without higher risk in hypertension: A systematic review and meta-analysis. World J. Urol. 2018;36:1285–1297. doi: 10.1007/s00345-018-2268-9. - DOI - PubMed
-
- Kelleher C., Hakimi Z., Zur R., Siddiqui E., Maman K., Aballéa S., Nazir J., Chapple C. Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: A systematic review and network meta-analysis. Eur. Urol. 2018;74:324–333. doi: 10.1016/j.eururo.2018.03.020. - DOI - PubMed
-
- Alessio B., Pietro A., Emanuele A. Crystalline Forms of Mirabegron Acetate Salt. 2-857389. EP Patent. 2015 August 4;
-
- FDA Clinical Pharmacology and Biopharmaceutics Review(s) Application Number: 202611Orig1s000. [(accessed on 7 March 2012)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202611Orig1s000C....
-
- Patel D.A., Patel D.J. Physicochemical characterization and in vitro dissolution enhancement of mirabegron using solid dispersion method. World J. Pharm. 2018;7:973–991.
Grants and funding
LinkOut - more resources
Full Text Sources

