Development and Optimization of a Novel Lozenge Containing a Metronidazole-Peppermint Oil-Tranexamic Acid Self-Nanoemulsified Delivery System to Be Used after Dental Extraction: In Vitro Evaluation and In Vivo Appraisal
- PMID: 37765310
- PMCID: PMC10535350
- DOI: 10.3390/pharmaceutics15092342
Development and Optimization of a Novel Lozenge Containing a Metronidazole-Peppermint Oil-Tranexamic Acid Self-Nanoemulsified Delivery System to Be Used after Dental Extraction: In Vitro Evaluation and In Vivo Appraisal
Abstract
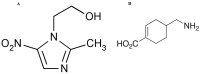
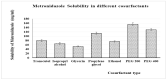
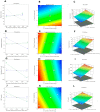
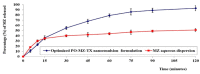
In-depth studies on essential oil-based nanoemulsions (NEs) have centered on a variety of oral health issues. NEs improve the delivery of nonpolar active agents to sites and thereby boost the dissolution and distribution of the agents. Metronidazole-peppermint oil-tranexamic acid self-nanoemulsifying drug delivery systems (MZ-PO-TX-SNEDDS) were created and loaded into novel lozenges to act as antifungal, hemostatic, antimicrobial, and analgesic dosage forms after dental extractions. The design-of-experiments approach was used in creating them. To generate the NEs, different concentrations of MZ-PO (240, 180, and 120 mg), 2% TX (600, 450, and 300 mg), and Smix1:1 (600, 400, and 200 mg) were used. The ideal formulation had serum levels of 1530 U/mL of interleukin-6, a minimal inhibitory concentration against bacteria of 1.5 µg/mL, a droplet size of 96 nm, and a blood coagulation time of 16.5 min. Moreover, the produced NE offered better MZ release. The adopted design was used to produce the ideal formulation; it contained 240 mg of MZ-PO, 600 mg of 2% TX, and 600 mg of Smix1:1. It was incorporated into lozenges with acceptable characteristics and an improved capability for drug release. These lozenges had reasonable coagulation times, IL-6 serum levels, and MIC values. All of these characteristics are desirable for managing symptoms following tooth extractions. Therefore, these lozenges loaded with MZ-PO-TX-SNEDDs might be considered a beneficial paradigm for relieving complications encountered after tooth extractions.
Keywords: hemostatic; lozenges; metronidazole; nanoemulsion; optimization; peppermint oil; periodontitis; tooth extraction; tranexamic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Preparation, Optimization, and Evaluation of Hyaluronic Acid-Based Hydrogel Loaded with Miconazole Self-Nanoemulsion for the Treatment of Oral Thrush.AAPS PharmSciTech. 2019 Aug 23;20(7):297. doi: 10.1208/s12249-019-1496-7. AAPS PharmSciTech. 2019. PMID: 31444661
-
Lyophilized Composite Loaded with Meloxicam-Peppermint oil Nanoemulsion for Periodontal Pain.Polymers (Basel). 2021 Jul 14;13(14):2317. doi: 10.3390/polym13142317. Polymers (Basel). 2021. PMID: 34301073 Free PMC article.
-
Tailoring and optimization of a honey-based nanoemulgel loaded with an itraconazole-thyme oil nanoemulsion for oral candidiasis.Drug Deliv. 2023 Dec;30(1):2173337. doi: 10.1080/10717544.2023.2173337. Drug Deliv. 2023. PMID: 36708105 Free PMC article.
-
Utilization of experimental design in the formulation and optimization of hyaluronic acid-based nanoemulgel loaded with a turmeric-curry leaf oil nanoemulsion for gingivitis.Drug Deliv. 2023 Dec;30(1):2184311. doi: 10.1080/10717544.2023.2184311. Drug Deliv. 2023. PMID: 36846914 Free PMC article.
-
Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances.Nanomedicine (Lond). 2010 Dec;5(10):1595-616. doi: 10.2217/nnm.10.126. Nanomedicine (Lond). 2010. PMID: 21143036 Review.
Cited by
-
Bioavailability Enhancement and Formulation Technologies of Oral Mucosal Dosage Forms: A Review.Pharmaceutics. 2025 Jan 22;17(2):148. doi: 10.3390/pharmaceutics17020148. Pharmaceutics. 2025. PMID: 40006515 Free PMC article. Review.
-
The pharmacokinetics of hexylresorcinol-containing lozenges and their antimicrobial efficacy against oral and respiratory microorganisms.J Oral Microbiol. 2025 Jul 7;17(1):2525229. doi: 10.1080/20002297.2025.2525229. eCollection 2025. J Oral Microbiol. 2025. PMID: 40635739 Free PMC article.
-
Advances in plant essential oils and drug delivery systems for skincare.Front Pharmacol. 2025 Apr 17;16:1578280. doi: 10.3389/fphar.2025.1578280. eCollection 2025. Front Pharmacol. 2025. PMID: 40313613 Free PMC article. Review.
References
-
- Passarelli P.C., Pagnoni S., Piccirillo G.B., Desantis V., Benegiamo M., Liguori A., Papa R., Papi P., Pompa G., D’Addona A. Reasons for Tooth Extractions and Related Risk Factors in Adult Patients: A Cohort Study. Int. J. Environ. Res. Public Health. 2020;17:2575. doi: 10.3390/ijerph17072575. - DOI - PMC - PubMed
-
- Beuling M.G., Agterbos P.C.G., van Riet P.C.T., Ho J.P.T.F., de Vries R., Kober J., de Lange J. Forces and movements during tooth extraction: A scoping review. Adv. Oral Maxillofacial Surg. 2023;9:100391. doi: 10.1016/j.adoms.2023.100391. - DOI
-
- Arteagoitia I., Ramos E., Santamaria G., Barbier L., Alvarez J., Santamaria J. Amoxicillin/clavulanic acid 2000/125 mg to prevent complications due to infection following completely bone-impacted lower third molar removal: A clinical trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015;119:8–16. doi: 10.1016/j.oooo.2014.08.014. - DOI - PubMed
LinkOut - more resources
Full Text Sources

