Cryopreservation of Duckweed Genetic Diversity as Model for Long-Term Preservation of Aquatic Flowering Plants
- PMID: 37765466
- PMCID: PMC10534739
- DOI: 10.3390/plants12183302
Cryopreservation of Duckweed Genetic Diversity as Model for Long-Term Preservation of Aquatic Flowering Plants
Abstract
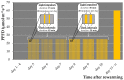
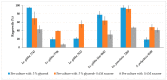
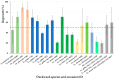
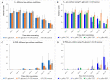
Vegetatively propagating aquatic angiosperms, the Lemnaceae family (duckweeds) represents valuable genetic resources for circular bioeconomics and other sustainable applications. Due to extremely fast growth and laborious cultivation of in vitro collections, duckweeds are an urgent subject for cryopreservation. We developed a robust and fast DMSO-free protocol for duckweed cryopreservation by vitrification. A single-use device was designed for sampling of duckweed fronds from donor culture, further spin-drying, and subsequent transferring to cryo-tubes with plant vitrification solution 3 (PVS3). Following cultivation in darkness and applying elevated temperatures during early regrowth stage, a specific pulsed illumination instead of a diurnal regime enabled successful regrowth after the cryopreservation of 21 accessions of Spirodela, Landoltia, Lemna, and Wolffia genera, including interspecific hybrids, auto- and allopolyploids. Genome size measurements revealed no quantitative genomic changes potentially caused by cryopreservation. The expression of CBF/DREB1 genes, considered as key factors in the development of freezing tolerance, was studied prior to cooling but was not linked with duckweed regrowth after rewarming. Despite preserving chlorophyll fluorescence after rewarming, the rewarmed fronds demonstrated nearly zero photosynthetic activity, which did not recover. The novel protocol provides the basis for future routine application of cryostorage to duckweed germplasm collections, saving labor for in vitro cultivation and maintaining characterized reference and mutant samples.
Keywords: CBF/DREB1 genes; PVS3; cryopreservation; duckweed; the operating efficiency of photosystem II; vitrification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Changes of soluble sugars and ATP content during DMSO droplet freezing and PVS3 droplet vitrification of potato shoot tips.Cryobiology. 2018 Dec;85:79-86. doi: 10.1016/j.cryobiol.2018.09.005. Epub 2018 Sep 23. Cryobiology. 2018. PMID: 30257179
-
Biodiversity of Duckweed (Lemnaceae) in Water Reservoirs of Ukraine and China Assessed by Chloroplast DNA Barcoding.Plants (Basel). 2022 May 30;11(11):1468. doi: 10.3390/plants11111468. Plants (Basel). 2022. PMID: 35684242 Free PMC article.
-
Limitation of current probe design for oligo-cross-FISH, exemplified by chromosome evolution studies in duckweeds.Chromosoma. 2021 Mar;130(1):15-25. doi: 10.1007/s00412-020-00749-2. Epub 2021 Jan 14. Chromosoma. 2021. PMID: 33443586 Free PMC article.
-
Genomes and Transcriptomes of Duckweeds.Front Chem. 2018 Jun 20;6:230. doi: 10.3389/fchem.2018.00230. eCollection 2018. Front Chem. 2018. PMID: 29974050 Free PMC article. Review.
-
Cryopreservation of Plant Genetic Resources.Adv Exp Med Biol. 2018;1081:355-369. doi: 10.1007/978-981-13-1244-1_19. Adv Exp Med Biol. 2018. PMID: 30288719 Review.
Cited by
-
7th International Conference on Duckweed Research and Applications: Depicting an Era of Advancing Research Translation Toward Practical Applications.Plants (Basel). 2025 Jul 11;14(14):2143. doi: 10.3390/plants14142143. Plants (Basel). 2025. PMID: 40733380 Free PMC article.
-
Duckweed: Beyond an Efficient Plant Model System.Biomolecules. 2024 May 27;14(6):628. doi: 10.3390/biom14060628. Biomolecules. 2024. PMID: 38927032 Free PMC article. Review.
References
-
- Tippery N.P., Les D.H. Tiny Plants with Enormous Potential: Phylogeny and Evolution of Duckweeds. In: Cao X.H., Fourounjian P., Wang W., editors. The Duckweed Genomes. Springer; Cham, Switzerland: 2020. pp. 19–38.
-
- Acosta K., Appenroth K.J., Borisjuk L., Edelman M., Heinig U., Jansen M.A.K., Oyama T., Pasaribu B., Schubert I., Sorrels S., et al. Return of the Lemnaceae: Duckweed as a model plant system in the genomics and postgenomics era. Plant Cell. 2021;33:3207–3234. doi: 10.1093/plcell/koab189. - DOI - PMC - PubMed
-
- Peterson A., Kishchenko O., Zhou Y., Vasylenko M., Giritch A., Sun J., Borisjuk N., Kuchuk M. Robust Agrobacterium-Mediated Transient Expression in Two Duckweed Species (Lemnaceae) Directed by Non-replicating, Replicating, and Cell-to-Cell Spreading Vectors. Front. Bioeng. Biotechnol. 2021;9:761073. doi: 10.3389/fbioe.2021.761073. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

