Polysaccharide/Carbon Quantum Dots Composite Film on Model Colloidal Particles-An Electro-Optical Study
- PMID: 37765620
- PMCID: PMC10536957
- DOI: 10.3390/polym15183766
Polysaccharide/Carbon Quantum Dots Composite Film on Model Colloidal Particles-An Electro-Optical Study
Abstract
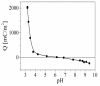
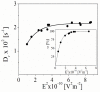
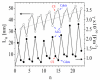
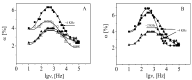
Negatively charged carbon dots (Cdots) were successfully impregnated into chitosan/alginate film formed on model colloidal particles as a result of the attractive interactions with the chitosan molecules. The electrical properties of the produced films were studied by electrokinetic spectroscopy. In this study, the electric light scattering method was applied for first the time for the investigation of suspensions of carbon-based structures. The electro-optical behavior for the suspension of polymer-coated particles showed that the electric polarizability of the particle-covered layer from alginate was significantly higher compared to that of the layer from chitosan due to the higher charge density of alginate. The presence of a low concentration of Cdots in the film results in partial charge screening. It was confirmed that the polarizability of counterions with lower mobility along the adsorbed polyion chains was responsible for the registered electro-optical effect from the suspension of polymer-coated particles and that the participation of diffuse H+ counterions of Cdots in the creation of the electro-optical effect was negligible. The observed oscillation behavior in the evolution of the film thickness was interpreted through the participation of compensatory effects due to the additional adsorption/desorption of polyelectrolyte complexes from the film surface. The concentration of Cdots in the film was determined, and the loaded amount was ca. 6.6 µg/mL per layer.
Keywords: carbon dots; electrokinetic spectroscopy; layer-by-layer assembly; polysaccharides.
Conflict of interest statement
The author declares no conflict of interest.
Figures






Similar articles
-
Comparative Electrokinetic Study of Alginate-Coated Colloidal Particles.Gels. 2023 Jun 16;9(6):493. doi: 10.3390/gels9060493. Gels. 2023. PMID: 37367163 Free PMC article.
-
Polymer concentration dependence of kilohertz electric polarizability of alumina colloid particles with adsorbed carboxymethyl cellulose.J Phys Condens Matter. 2010 Dec 15;22(49):494112. doi: 10.1088/0953-8984/22/49/494112. J Phys Condens Matter. 2010. PMID: 21406778
-
Electrical properties of polyelectrolyte layers adsorbed on colloidal particles at different ionic strength.Langmuir. 2010 Sep 21;26(18):14488-93. doi: 10.1021/la102428k. Langmuir. 2010. PMID: 20735019
-
Polymer adsorption and electrokinetic potential of dispersed particles in weak and strong electric fields.Adv Colloid Interface Sci. 2015 Aug;222:58-69. doi: 10.1016/j.cis.2014.09.009. Epub 2014 Nov 18. Adv Colloid Interface Sci. 2015. PMID: 25456453 Review.
-
Dynamics of polyelectrolyte adsorption and colloidal flocculation upon mixing studied using mono-dispersed polystyrene latex particles.Adv Colloid Interface Sci. 2015 Dec;226(Pt A):101-14. doi: 10.1016/j.cis.2015.09.004. Epub 2015 Sep 25. Adv Colloid Interface Sci. 2015. PMID: 26456137 Review.
References
-
- Fu X., Hosta-Rigau L., Chandrawati R., Cyu J. Multi-stimuli-responsive polymer particles, films, and hydrogels for drug delivery. Chem. Cell Press. 2018;4:2084–2107. doi: 10.1016/j.chempr.2018.07.002. - DOI
-
- Decher J.D., Hong J., Schmitt J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Sol. Film. 1992;210/211:831–835. doi: 10.1016/0040-6090(92)90417-A. - DOI
LinkOut - more resources
Full Text Sources

