Improving Sustainability through Covalent Adaptable Networks in the Recycling of Polyurethane Plastics
- PMID: 37765634
- PMCID: PMC10537520
- DOI: 10.3390/polym15183780
Improving Sustainability through Covalent Adaptable Networks in the Recycling of Polyurethane Plastics
Abstract
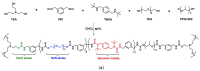
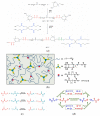
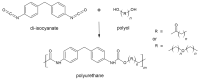
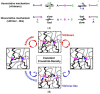
The global plastic waste problem has created an urgent need for the development of more sustainable materials and recycling processes. Polyurethane (PU) plastics, which represent 5.5% of globally produced plastics, are particularly challenging to recycle owing to their crosslinked structure. Covalent adaptable networks (CANs) based on dynamic covalent bonds have emerged as a promising solution for recycling PU waste. CANs enable the production of thermoset polymers that can be recycled using methods that are traditionally reserved for thermoplastic polymers. Reprocessing using hot-pressing techniques, in particular, proved to be more suited for the class of polyurethanes, allowing for the efficient recycling of PU materials. This Review paper explores the potential of CANs for improving the sustainability of PU recycling processes by examining different types of PU-CANs, bond types, and fillers that can be used to optimise the recycling efficiency. The paper concludes that further research is needed to develop more cost-effective and industrial-friendly techniques for recycling PU-CANs, as they can significantly contribute to sustainable development by creating recyclable thermoset polymers.
Keywords: CAN; composites; mechanical recycling; poly(urethane-urea); polyhydroxyurethane; polythiourethanes; polyurethanes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Plastics-the Facts 2022 OCTOBER 2022. [(accessed on 15 June 2023)]. Available online: https://plasticseurope.org/
-
- Otto B. Das Di-Isocyanat-Polyadditionsverfahren (Polyurethane) Angew. Chem. 1947;59:257–288.
-
- Akindoyo J.O., Beg M.D.H., Ghazali S., Islam M.R., Jeyaratnam N., Yuvaraj A.R. Polyurethane types, synthesis and applications-a review. RSC Adv. 2016;6:114453–114482. doi: 10.1039/C6RA14525F. - DOI
Publication types
LinkOut - more resources
Full Text Sources

