Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells
- PMID: 37765637
- PMCID: PMC10536614
- DOI: 10.3390/polym15183783
Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells
Abstract
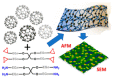
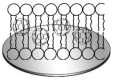
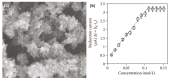
Conductive polymers and their composites are excellent materials for coupling biological materials and electrodes in bioelectrochemical systems. It is assumed that their relevance and introduction to the field of bioelectrochemical devices will only grow due to their tunable conductivity, easy modification, and biocompatibility. This review analyzes the main trends and trends in the development of the methodology for the application of conductive polymers and their use in biosensors and biofuel elements, as well as describes their future prospects. Approaches to the synthesis of such materials and the peculiarities of obtaining their nanocomposites are presented. Special emphasis is placed on the features of the interfaces of such materials with biological objects.
Keywords: bioelectrochemistry; biosensors; conducting polymers; electrochemical sensors; microbial and enzymatic biofuel cells; nanocomposites; polymer-modified electrodes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Conducting Polymers in the Design of Biosensors and Biofuel Cells.Polymers (Basel). 2020 Dec 25;13(1):49. doi: 10.3390/polym13010049. Polymers (Basel). 2020. PMID: 33375584 Free PMC article. Review.
-
Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells.Nanomaterials (Basel). 2021 Feb 2;11(2):371. doi: 10.3390/nano11020371. Nanomaterials (Basel). 2021. PMID: 33540587 Free PMC article. Review.
-
Recent Progress in the Development of Conducting Polymer-Based Nanocomposites for Electrochemical Biosensors Applications: A Mini-Review.Chem Rec. 2018 Jun;18(6):599-618. doi: 10.1002/tcr.201700101. Epub 2018 Feb 20. Chem Rec. 2018. PMID: 29460399 Review.
-
Recent advances in conductive polymer hydrogel composites and nanocomposites for flexible electrochemical supercapacitors.Chem Commun (Camb). 2021 Dec 23;58(2):185-207. doi: 10.1039/d1cc05526g. Chem Commun (Camb). 2021. PMID: 34881748 Review.
-
Intrinsically Conductive Polymer Nanocomposites for Cellular Applications.Adv Exp Med Biol. 2018;1078:135-153. doi: 10.1007/978-981-13-0950-2_8. Adv Exp Med Biol. 2018. PMID: 30357622 Review.
Cited by
-
A "2-in-1" Bioanalytical System Based on Nanocomposite Conductive Polymers for Early Detection of Surface Water Pollution.Polymers (Basel). 2024 May 17;16(10):1431. doi: 10.3390/polym16101431. Polymers (Basel). 2024. PMID: 38794624 Free PMC article.
-
Whole Cells of Microorganisms-A Powerful Bioanalytical Tool for Measuring Integral Parameters of Pollution: A Review.Biosensors (Basel). 2025 May 4;15(5):290. doi: 10.3390/bios15050290. Biosensors (Basel). 2025. PMID: 40422029 Free PMC article. Review.
-
Bionanocomposite Four-Channel Biosensor for Rapid and Convenient Monitoring of Glucose, Lactate, Ethanol and Starch.Gels. 2025 May 12;11(5):355. doi: 10.3390/gels11050355. Gels. 2025. PMID: 40422375 Free PMC article.
-
Recent Advances in Enzymatic Biofuel Cells to Power Up Wearable and Implantable Biosensors.Biosensors (Basel). 2025 Mar 28;15(4):218. doi: 10.3390/bios15040218. Biosensors (Basel). 2025. PMID: 40277532 Free PMC article. Review.
-
Microbial Biofilms: Features of Formation and Potential for Use in Bioelectrochemical Devices.Biosensors (Basel). 2024 Jun 8;14(6):302. doi: 10.3390/bios14060302. Biosensors (Basel). 2024. PMID: 38920606 Free PMC article. Review.
References
-
- Pal T., Banerjee S., Manna P.K., Kar K.K. Characteristics of Conducting Polymers BT. In: Kar K.K., editor. Handbook of Nanocomposite Supercapacitor Materials I: Characteristics. Springer International Publishing; Cham, Switzerland: 2020. pp. 247–268.
-
- Shirakawa H., Louis E.J., MacDiarmid A.G., Chiang C.K., Heeger A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene,(CH) x. J. Chem. Soc. Chem. Commun. 1977;16:578–580. doi: 10.1039/c39770000578. - DOI
-
- Kanazawa K.K., Diaz A.F., Geiss R.H., Gill W.D., Kwak J.F., Logan J.A., Rabolt J.F., Street G.B. ‘Organic metals’: Polypyrrole, a stable synthetic ‘metallic’polymer. J. Chem. Soc. Chem. Commun. 1979;19:854–855. doi: 10.1039/C39790000854. - DOI
-
- Park Y., Jung J., Chang M. Research progress on conducting polymer-based biomedical applications. Appl. Sci. 2019;9:1070. doi: 10.3390/app9061070. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

