Screening of Oligomeric (Meth)acrylate Vaccine Adjuvants Synthesized via Catalytic Chain Transfer Polymerization
- PMID: 37765685
- PMCID: PMC10538096
- DOI: 10.3390/polym15183831
Screening of Oligomeric (Meth)acrylate Vaccine Adjuvants Synthesized via Catalytic Chain Transfer Polymerization
Abstract
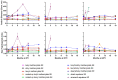
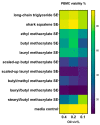
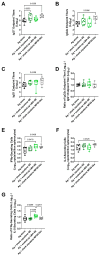
This report details the first systematic screening of free-radical-produced methacrylate oligomer reaction mixtures as alternative vaccine adjuvant components to replace the current benchmark compound squalene, which is unsustainably sourced from shark livers. Homo-/co-oligomer mixtures of methyl, butyl, lauryl, and stearyl methacrylate were successfully synthesized using catalytic chain transfer control, where the use of microwave heating was shown to promote propagation over chain transfer. Controlling the mixture material properties allowed the correct viscosity to be achieved, enabling the mixtures to be effectively used in vaccine formulations. Emulsions of selected oligomers stimulated comparable cytokine levels to squalene emulsion when incubated with human whole blood and elicited an antigen-specific cellular immune response when administered with an inactivated influenza vaccine, indicating the potential utility of the compounds as vaccine adjuvant components. Furthermore, the oligomers' molecular sizes were demonstrated to be large enough to enable greater emulsion stability than squalene, especially at high temperatures, but are predicted to be small enough to allow for rapid clearance from the body.
Keywords: adjuvant; catalytic chain transfer; polymerization; screening; squalene; vaccine.
Conflict of interest statement
C.B.F., D.J.I., and C.S.H. are inventors in patents and/or patent applications relating to the synthesis of novel oligomeric (meth)acrylate vaccine adjuvants via catalytic chain transfer polymerization. All other authors declare they have no conflicts of interest.
Figures

References
-
- Gorse G.J., Grimes S., Buck H., Mulla H., White P., Hill H., May J., Frey S.E., Blackburn P. A Phase 1 Dose-Sparing, Randomized Clinical Trial of Seasonal Trivalent Inactivated Influenza Vaccine Combined with MAS-1, a Novel Water-in-Oil Adjuvant/Delivery System. Vaccine. 2022;40:1271–1281. doi: 10.1016/j.vaccine.2022.01.034. - DOI - PubMed
-
- Fox C.B., Anderson R.C., Dutill T.S., Goto Y., Reed S.G., Vedvick T.S. Monitoring the Effects of Component Structure and Source on Formulation Stability and Adjuvant Activity of Oil-in-Water Emulsions. Colloids Surf. B Biointerfaces. 2008;65:98–105. doi: 10.1016/j.colsurfb.2008.03.003. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

