Advancements in Chitosan-Based Nanoparticles for Pulmonary Drug Delivery
- PMID: 37765701
- PMCID: PMC10536410
- DOI: 10.3390/polym15183849
Advancements in Chitosan-Based Nanoparticles for Pulmonary Drug Delivery
Abstract
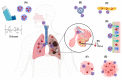
The evolution of respiratory diseases represents a considerable public health challenge, as they are among the leading causes of death worldwide. In this sense, in addition to the high prevalence of diseases such as asthma, chronic obstructive pulmonary disease, pneumonia, cystic fibrosis, and lung cancer, emerging respiratory diseases, particularly those caused by members of the coronavirus family, have contributed to a significant number of deaths on a global scale over the last two decades. Therefore, several studies have been conducted to optimize the efficacy of treatments against these diseases, focusing on pulmonary drug delivery using nanomedicine. Thus, the development of nanocarriers has emerged as a promising alternative to overcome the limitations of conventional therapy, by increasing drug bioavailability at the target site and reducing unwanted side effects. In this context, nanoparticles composed of chitosan (CS) show advantages over other nanocarriers because chitosan possesses intrinsic biological properties, such as anti-inflammatory, antimicrobial, and mucoadhesive capacity. Moreover, CS nanoparticles have the potential to enhance drug stability, prolong the duration of action, improve drug targeting, control drug release, optimize dissolution of poorly soluble drugs, and increase cell membrane permeability of hydrophobic drugs. These properties could optimize the performance of the drug after its pulmonary administration. Therefore, this review aims to discuss the potential of chitosan nanoparticles for pulmonary drug delivery, highlighting how their biological properties can improve the treatment of pulmonary diseases, including their synergistic action with the encapsulated drug.
Keywords: chitosan; chitosan derivatives; lung delivery; lung diseases; nanoparticles; pulmonary drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The Role of Inhaled Chitosan-Based Nanoparticles in Lung Cancer Therapy.Pharmaceutics. 2024 Jul 23;16(8):969. doi: 10.3390/pharmaceutics16080969. Pharmaceutics. 2024. PMID: 39204314 Free PMC article. Review.
-
Application of Chitosan and its Derivatives in Nanocarrier Based Pulmonary Drug Delivery Systems.Pharm Nanotechnol. 2017;5(4):243-249. doi: 10.2174/2211738505666170808095258. Pharm Nanotechnol. 2017. PMID: 28786352 Review.
-
Mesoporous silica nanoparticles for pulmonary drug delivery.Adv Drug Deliv Rev. 2021 Oct;177:113953. doi: 10.1016/j.addr.2021.113953. Epub 2021 Aug 30. Adv Drug Deliv Rev. 2021. PMID: 34474094 Review.
-
Recent advances in chitosan-based nanoparticulate pulmonary drug delivery.Nanoscale. 2016 Aug 14;8(30):14341-58. doi: 10.1039/c6nr03256g. Epub 2016 Jul 20. Nanoscale. 2016. PMID: 27439116 Review.
-
Eco-friendly chitosan-based nanostructures in diabetes mellitus therapy: Promising bioplatforms with versatile therapeutic perspectives.Environ Res. 2023 Jul 1;228:115912. doi: 10.1016/j.envres.2023.115912. Epub 2023 Apr 15. Environ Res. 2023. PMID: 37068723 Review.
Cited by
-
Ferroptosis in Pulmonary Disease and Lung Cancer: Molecular Mechanisms, Crosstalk Regulation, and Therapeutic Strategies.MedComm (2020). 2025 Feb 23;6(3):e70116. doi: 10.1002/mco2.70116. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 39991627 Free PMC article. Review.
-
Microfluidic Synthesis of Scalable Layer-by-Layer Multiple Antigen Nano-Delivery Platform for SARS-CoV-2 Vaccines.Vaccines (Basel). 2024 Mar 21;12(3):339. doi: 10.3390/vaccines12030339. Vaccines (Basel). 2024. PMID: 38543973 Free PMC article.
-
Hygroscopic properties modeling and thermodynamic analysis of a seamless popping capsule.Sci Rep. 2025 Aug 17;15(1):30041. doi: 10.1038/s41598-025-15452-4. Sci Rep. 2025. PMID: 40820119 Free PMC article.
-
The Role of Inhaled Chitosan-Based Nanoparticles in Lung Cancer Therapy.Pharmaceutics. 2024 Jul 23;16(8):969. doi: 10.3390/pharmaceutics16080969. Pharmaceutics. 2024. PMID: 39204314 Free PMC article. Review.
-
Comprehensive Aerodynamic and Physicochemical Stability Evaluations of Nanocrystal-Based Dry Powder Inhalers: The Role of Mannitol and Leucine in Enhancing Performance.Pharmaceutics. 2025 Mar 28;17(4):436. doi: 10.3390/pharmaceutics17040436. Pharmaceutics. 2025. PMID: 40284431 Free PMC article.
References
-
- Aveyard P., Gao M., Lindson N., Hartmann-Boyce J., Watkinson P., Young D., Coupland C.A.C., Tan P.S., Clift A.K., Harrison D., et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: A population cohort study. Lancet Respir. Med. 2021;9:909–923. doi: 10.1016/S2213-2600(21)00095-3. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

