Anti-S and Anti-N Antibody Responses of COVID-19 Vaccine Recipients
- PMID: 37766076
- PMCID: PMC10537031
- DOI: 10.3390/vaccines11091398
Anti-S and Anti-N Antibody Responses of COVID-19 Vaccine Recipients
Abstract
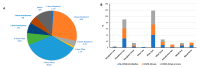
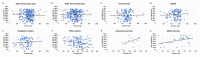
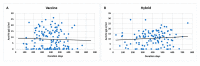
The long-term immunoglobulin responses of COVID-19 vaccinations is important to determine the efficacy of these vaccinations. This study aimed to investigate and compare the long-term immunoglobulin response of COVID-19 vaccination recipients, using anti-S IgG, anti-N IgG, and IgM titer levels. This study included 267 participants, comprising individuals who tested positive for COVID-19 through PCR testing (n = 125), and those who received the Pfizer (n = 133), Sinopharm (n = 112), AstraZeneca (n = 20), or Sputnik (n = 2) vaccines. Female participants comprised the largest share of this study (n = 147, 55.1%). This study found that most participants had positive IgG antibodies, with 96.3% having anti-S IgG and 75.7% having anti-N IgG. Most participants (90.3%) tested negative for anti-N IgM antibodies. Sinopharm-vaccinated individuals exhibited a notably lower rate of positive anti-S IgG (93.8%) and a significantly higher rate of positive anti-N IgG antibodies (91%). Anti-N IgG levels were significantly correlated with the number of prior COVID-19 infections (p = 0.015). Specifically, individuals with a history of four COVID-19 infections had higher anti-N IgG titers (14.1 ± 1.4) than those with only one experience of COVID-19 infection (9.4 ± 7.2). Individuals who were infected with COVID-19 after receiving the vaccine demonstrated higher levels of anti-N IgG, exhibiting a 25% increase in mean titer levels compared to those who were infected prior to vaccination. There was a statistically significant association between anti-N IgG positivity with age (p = 0.034), and smoking status (p = 0.006) of participants. Participants younger than 20 and older than 60 showed the highest positivity rate of anti-N (>90%). Smokers had a low positivity rate of anti-N (68.8%) compared to nonsmokers (83.6%). In conclusion, this study demonstrated that most COVID-19 vaccination recipients had positive IgG antibodies, with differences in the long-term immunoglobulin response depending on the type of vaccine administered and occurrence of COVID-19 infection.
Keywords: AstraZeneca; COVID-19 vaccines; Pfizer–BioNTech; Sinopharm; anti-N; anti-S; antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- WHO Archived: WHO Timeline-COVID-19. [(accessed on 10 March 2023)]. Available online: https://www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19.
Grants and funding
LinkOut - more resources
Full Text Sources

