Oral Immunization with rVSV Bivalent Vaccine Elicits Protective Immune Responses, Including ADCC, against Both SARS-CoV-2 and Influenza A Viruses
- PMID: 37766083
- PMCID: PMC10534613
- DOI: 10.3390/vaccines11091404
Oral Immunization with rVSV Bivalent Vaccine Elicits Protective Immune Responses, Including ADCC, against Both SARS-CoV-2 and Influenza A Viruses
Abstract
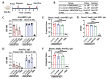
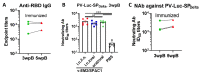
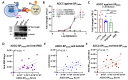
COVID-19 and influenza both cause enormous disease burdens, and vaccines are the primary measures for their control. Since these viral diseases are transmitted through the mucosal surface of the respiratory tract, developing an effective and convenient mucosal vaccine should be a high priority. We previously reported a recombinant vesicular stomatitis virus (rVSV)-based bivalent vaccine (v-EM2/SPΔC1Delta) that protects animals from both SARS-CoV-2 and influenza viruses via intramuscular and intranasal immunization. Here, we further investigated the immune response induced by oral immunization with this vaccine and its protective efficacy in mice. The results demonstrated that the oral delivery, like the intranasal route, elicited strong and protective systemic immune responses against SARS-CoV-2 and influenza A virus. This included high levels of neutralizing antibodies (NAbs) against SARS-CoV-2, as well as strong anti-SARS-CoV-2 spike protein (SP) antibody-dependent cellular cytotoxicity (ADCC) and anti-influenza M2 ADCC responses in mice sera. Furthermore, it provided efficient protection against challenge with influenza H1N1 virus in a mouse model, with a 100% survival rate and a significantly low lung viral load of influenza virus. All these findings provide substantial evidence for the effectiveness of oral immunization with the rVSV bivalent vaccine.
Keywords: ADCC activity; COVID-19; SARS-CoV-2; VSV vector; bivalent vaccine; influenza virus; neutralizing antibody; oral immunization; vesicular stomatitis virus (VSV).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Buchan S.A., Chung H., Brown K.A., Austin P.C., Fell D.B., Gubbay J.B., Nasreen S., Schwartz K.L., Sundaram M.E., Tadrous M., et al. Estimated Effectiveness of COVID-19 Vaccines Against Omicron or Delta Symptomatic Infection and Severe Outcomes. JAMA Netw. Open. 2022;5:e2232760. doi: 10.1001/jamanetworkopen.2022.32760. - DOI - PMC - PubMed
-
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. [(accessed on 15 June 2023)];2023 Available online: https://covid19.who.int/table.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

