Inhibition of Trichinella spiralis Membrane-Associated Progesterone Receptor (MAPR) Results in a Reduction in Worm Burden
- PMID: 37766114
- PMCID: PMC10535220
- DOI: 10.3390/vaccines11091437
Inhibition of Trichinella spiralis Membrane-Associated Progesterone Receptor (MAPR) Results in a Reduction in Worm Burden
Abstract
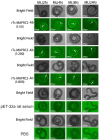
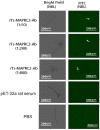
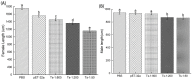
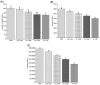
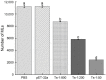
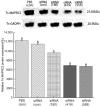
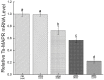
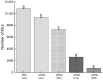
Trichinella spiralis (T. spiralis), a nematode parasite, is the major cause of Trichinellosis, a zoonotic disease. A key role of MAPR in the reproductive system is to maintain pregnancy. Previous studies found that antihormone drug design and vaccine therapy of recombinant protein (rTs-MAPRC2) control T. spiralis infection. The current study investigates the inhibitory effects of different ratios of antibodies against Ts-MAPRC2 on the development of muscle larvae (ML) and newborn larvae (NBL). First, we performed indirect immunofluorescence assays and examined the effects of rTs-MAPRC2-Ab on ML and NBL in vitro as well as in vivo. Afterward, siRNA-Ts-MAPRC2 was transfected into T. spiralis muscle larvae. Following that, Ts-MAPRC2 protein was detected by Western Blotting, and mRNA levels were determined by qPCR. We also assessed whether siRNA-treated NBLs were infective by analyzing muscle larvae burden (MLs). Our results showed that rTs-MAPRC2-Ab greatly inhibited the activity of the Ts-MAPRC2 in ML and NBL of T. spiralis and rTs-MAPRC2-Ab reduced larval infectivity and survival in the host in a dose-dependent manner (1:50, 1:200, 1:800 dilutions). Furthermore, siRNA-Ts-MAPRC2 effectively silenced the Ts-MAPRC2 gene in muscle larvae (ML) in vitro, as well as in newborn larvae (NBL) of T. spiralis in vivo. In addition, siRNA-Ts-MAPRC2 (siRNA180, siRNA419, siRNA559) reduced host larval survival and infectivity significantly. This study, therefore, suggests that Ts-MAPRC2 might be a novel molecular target useful in the development of vaccines against T. spiralis infection.
Keywords: Trichinella spiralis; inhibitory effect; knockdown; rTs-MAPRC2-Ab; worm burden.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Characterization of Membrane-Associated Progesterone Receptor Component-2 (MAPRC2) from Trichinella spiralis and Its Interaction with Progesterone and Mifepristone.Vaccines (Basel). 2021 Aug 23;9(8):934. doi: 10.3390/vaccines9080934. Vaccines (Basel). 2021. PMID: 34452060 Free PMC article.
-
Molecular Docking and In Silico Simulation of Trichinella spiralis Membrane-Associated Progesterone Receptor Component 2 (Ts-MAPRC2) and Its Interaction with Human PGRMC1.Biomed Res Int. 2022 Jun 20;2022:7414198. doi: 10.1155/2022/7414198. eCollection 2022. Biomed Res Int. 2022. PMID: 35769668 Free PMC article.
-
Molecular Characterization of Fructose-1,6-bisphosphate Aldolase From Trichinella spiralis and Its Potential in Inducing Immune Protection.Front Cell Infect Microbiol. 2019 Apr 24;9:122. doi: 10.3389/fcimb.2019.00122. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31069178 Free PMC article.
-
Partially Protective Immunity Induced by a 20 kDa Protein Secreted by Trichinella spiralis Stichocytes.PLoS One. 2015 Aug 19;10(8):e0136189. doi: 10.1371/journal.pone.0136189. eCollection 2015. PLoS One. 2015. PMID: 26288365 Free PMC article.
-
Trichinella spiralis: genomic application to control a zoonotic nematode.Infect Disord Drug Targets. 2010 Oct;10(5):376-84. doi: 10.2174/187152610793180830. Infect Disord Drug Targets. 2010. PMID: 20701572 Review.
Cited by
-
Alendronate repositioning as potential anti-parasitic agent targeting Trichinella spiralis inorganic pyrophosphatase, in vitro supported molecular docking and molecular dynamics simulation study.BMC Chem. 2025 May 6;19(1):119. doi: 10.1186/s13065-025-01468-4. BMC Chem. 2025. PMID: 40329381 Free PMC article.
-
Steroid hormone binding and modulation of Trichinella spiralis progesterone receptor: A computational approach.Biochem Biophys Rep. 2025 May 5;42:102031. doi: 10.1016/j.bbrep.2025.102031. eCollection 2025 Jun. Biochem Biophys Rep. 2025. PMID: 40458199 Free PMC article.
References
-
- U.N. Food & Agriculture Organization . World Health Organization Multicriteria-Based Ranking for Risk Management of Foodborne Parasites [Preliminary Report] FAO; Rome, Italy: 2012. p. 47.
LinkOut - more resources
Full Text Sources
Research Materials

