Efficacy, Immunogenicity, and Safety of COVID-19 Vaccines in Patients with Autoimmune Diseases: A Systematic Review and Meta-Analysis
- PMID: 37766132
- PMCID: PMC10535431
- DOI: 10.3390/vaccines11091456
Efficacy, Immunogenicity, and Safety of COVID-19 Vaccines in Patients with Autoimmune Diseases: A Systematic Review and Meta-Analysis
Abstract
Patients with autoimmune diseases are among the susceptible groups to COVID-19 infection because of the complexity of their conditions and the side effects of the immunosuppressive drugs used to treat them. They might show impaired immunogenicity to COVID-19 vaccines and have a higher risk of developing COVID-19. Using a systematic review and meta-analysis, this research sought to summarize the evidence on COVID-19 vaccine efficacy, immunogenicity, and safety in patients with autoimmune diseases following predefined eligibility criteria. Research articles were obtained from an initial search up to 26 September 2022 from PubMed, Embase, EBSCOhost, ProQuest, MedRxiv, bioRxiv, SSRN, EuroPMC, and the Cochrane Center of Randomized Controlled Trials (CCRCT). Of 76 eligible studies obtained, 29, 54, and 38 studies were included in systematic reviews of efficacy, immunogenicity, and safety, respectively, and 6, 18, and 4 studies were included in meta-analyses for efficacy, immunogenicity, and safety, respectively. From the meta-analyses, patients with autoimmune diseases showed more frequent breakthrough COVID-19 infections and lower total antibody (TAb) titers, IgG seroconversion, and neutralizing antibodies after inactivated COVID-19 vaccination compared with healthy controls. They also had more local and systemic adverse events after the first dose of inactivated vaccination compared with healthy controls. After COVID-19 mRNA vaccination, patients with autoimmune diseases had lower TAb titers and IgG seroconversion compared with healthy controls.
Keywords: COVID-19; autoimmune; efficacy; immunogenicity; safety; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

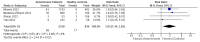











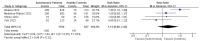
References
-
- WHO WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. 2022. [(accessed on 3 May 2022)]. Available online: https://covid19.who.int/
-
- Centers for Disease Control Prevention (CDC) People with Certain Medical, Conditions|CDC. [(accessed on 2 July 2022)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

