Comparative Safety and Effectiveness of Heterologous CoronaVac-ChAdOx1 versus Homologous CoronaVac Vaccination in a Real-World Setting: A Retrospective Cohort Study
- PMID: 37766134
- PMCID: PMC10535109
- DOI: 10.3390/vaccines11091458
Comparative Safety and Effectiveness of Heterologous CoronaVac-ChAdOx1 versus Homologous CoronaVac Vaccination in a Real-World Setting: A Retrospective Cohort Study
Abstract
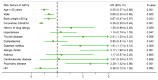
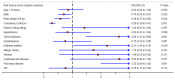
Background: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has outpaced vaccine availability and delivery from vaccine manufacturers, and thus, a scarcity of vaccines happened to many countries around the world. In Thailand, the mixing of different types of vaccines was approved and clinically implemented partially due to concerns about the availability and efficacy of one vaccine. Objective: This study aimed to investigate the effectiveness and safety of heterologous CoronaVac-ChAdOx1 nCoV-19 vaccines compared with the usual regimen of homologous CoronaVac-CoronaVac. A retrospective cohort study was conducted by dividing patients into the CoronaVac-CoronaVac group and the CoronaVac-ChAdOx1 group. Results: A total of 875 patients received vaccinations at Srisangwan Hospital between April to October 2021 and were included for analysis. The patients in both homologous and heterologous groups had low rates of COVID-19 infection. In addition, the hospitalization rates in the 40 days after the second vaccination were low in both regimens. Minimal adverse events (AE) were reported in both groups, including local AE (e.g., discomfort at the injection site, rash, soreness, swelling, and redness) and systemic AE (e.g., fever, headache, weariness, nausea, vomiting, diarrhoea, and myalgia). Moreover, several factors were associated with lower adverse events following immunization (AEFIs), including age ≥ 50 years, male, and body weight ≥ 50 kg. In contrast, thyroid disease, diabetes mellitus, allergic rhinitis, and psychiatric disorders were independent risk factors associated with an increase in AEFIs. Conclusions: The heterologous CoronaVac-ChAdOx1 and homologous CoronaVac-CoronaVac regimens were promising vaccination strategies for the prevention of SARS-CoV-2 infection. However, the heterologous CoronaVac-ChAdOx1 potentially caused fewer AEFIs compared with the homologous CoronaVac-CoronaVac regimen.
Keywords: ChAdOx1; CoronaVac; heterologous vaccine regimen; homologous vaccine regimen; real-world setting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Durability of the Effectiveness of Heterologous COVID-19 Vaccine Regimens in Thailand: Retrospective Cohort Study Using National Registration Data.JMIR Public Health Surveill. 2024 Mar 5;10:e48255. doi: 10.2196/48255. JMIR Public Health Surveill. 2024. PMID: 38441923 Free PMC article.
-
The Pilot Study of Immunogenicity and Adverse Events of a COVID-19 Vaccine Regimen: Priming with Inactivated Whole SARS-CoV-2 Vaccine (CoronaVac) and Boosting with the Adenoviral Vector (ChAdOx1 nCoV-19) Vaccine.Vaccines (Basel). 2022 Mar 30;10(4):536. doi: 10.3390/vaccines10040536. Vaccines (Basel). 2022. PMID: 35455285 Free PMC article.
-
Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdOx1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: a randomised, observer-masked, controlled trial in Indonesia.Lancet Infect Dis. 2023 May;23(5):545-555. doi: 10.1016/S1473-3099(22)00800-3. Epub 2023 Jan 11. Lancet Infect Dis. 2023. PMID: 36640798 Clinical Trial.
-
Anti-SARS-CoV-2 spike IgG following injection of the third dose vaccine: A systematic review with meta-analysis of heterologous versus homologous vaccination.Front Public Health. 2023 Jan 12;10:960598. doi: 10.3389/fpubh.2022.960598. eCollection 2022. Front Public Health. 2023. PMID: 36711369 Free PMC article.
-
Binding and neutralizing antibody levels and vaccine efficacy/effectiveness compared between heterologous and homologous primary series COVID-19 vaccination: A systematic review and meta-analysis.Asian Pac J Allergy Immunol. 2022 Dec;40(4):321-336. doi: 10.12932/AP-121122-1501. Asian Pac J Allergy Immunol. 2022. PMID: 36681658
Cited by
-
Effectiveness of different booster vaccine combinations against SARS-CoV-2 during a six-month follow-up in Mexico and Argentina.Front Immunol. 2024 May 14;15:1403784. doi: 10.3389/fimmu.2024.1403784. eCollection 2024. Front Immunol. 2024. PMID: 38807602 Free PMC article.
References
-
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

