Unconjugated Multi-Epitope Peptides Adjuvanted with ALFQ Induce Durable and Broadly Reactive Antibodies to Human and Avian Influenza Viruses
- PMID: 37766144
- PMCID: PMC10537791
- DOI: 10.3390/vaccines11091468
Unconjugated Multi-Epitope Peptides Adjuvanted with ALFQ Induce Durable and Broadly Reactive Antibodies to Human and Avian Influenza Viruses
Abstract
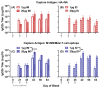
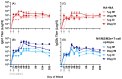
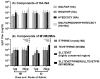
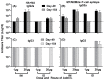
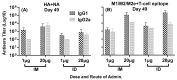
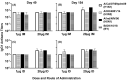
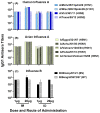
An unconjugated composite peptide vaccine targeting multiple conserved influenza epitopes from hemagglutinin, neuraminidase, and matrix protein and formulated with a safe and highly potent adjuvant, Army Liposome formulation (ALFQ), generated broad and durable immune responses in outbred mice. The antibodies recognized specific epitopes in influenza peptides and several human, avian, and swine influenza viruses. Comparable antibody responses to influenza viruses were observed with intramuscular and intradermal routes of vaccine administration. The peptide vaccine induced cross-reactive antibodies that recognized influenza virus subtypes A/H1N1, A/H3N2, A/H5N1, B/Victoria, and B/Yamagata. In addition, immune sera neutralized seasonal and pandemic influenza strains (Group 1 and Group 2). This composite multi-epitope peptide vaccine, formulated with ALFQ and administered via intramuscular and intradermal routes, provides a high-performance supra-seasonal vaccine that would be cost-effective and easily scalable, thus moving us closer to a viable strategy for a universal influenza vaccine and pandemic preparedness.
Keywords: ALFQ; QS21 (also known as QS-21); broadly reactive antibodies; durable; immune responses; influenza; intradermal; intramuscular; liposomes; neutralizing antibodies; peptides; universal vaccine.
Conflict of interest statement
Gerald W. Fischer and Clara J. Sei are inventors on patents for composite influenza peptide vaccines. Carl R. Alving is an inventor on a U.S. patent for ALFQ. Carl R. Alving and Gary R. Matyas are inventors on a U.S. patent for a malaria vaccine formulated with ALFQ. The other authors declare no conflict of interest.
Figures


Similar articles
-
Conserved Influenza Hemagglutinin, Neuraminidase and Matrix Peptides Adjuvanted with ALFQ Induce Broadly Neutralizing Antibodies.Vaccines (Basel). 2021 Jun 25;9(7):698. doi: 10.3390/vaccines9070698. Vaccines (Basel). 2021. PMID: 34202178 Free PMC article.
-
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article.
-
Highly conserved sequences for human neutralization epitope on hemagglutinin of influenza A viruses H3N2, H1N1 and H5N1: Implication for human monoclonal antibody recognition.Biochem Biophys Res Commun. 2010 Mar 19;393(4):614-8. doi: 10.1016/j.bbrc.2010.02.031. Epub 2010 Feb 10. Biochem Biophys Res Commun. 2010. PMID: 20152806
-
One step closer to universal influenza epitopes.Expert Rev Anti Infect Ther. 2009 Aug;7(6):687-90. doi: 10.1586/eri.09.48. Expert Rev Anti Infect Ther. 2009. PMID: 19681695 Review.
-
MF59-adjuvanted vaccines for seasonal and pandemic influenza prophylaxis.Influenza Other Respir Viruses. 2008 Nov;2(6):243-9. doi: 10.1111/j.1750-2659.2008.00059.x. Influenza Other Respir Viruses. 2008. PMID: 19453401 Free PMC article. Review.
Cited by
-
Research Progress of Universal Influenza Vaccine.Vaccines (Basel). 2025 Aug 15;13(8):863. doi: 10.3390/vaccines13080863. Vaccines (Basel). 2025. PMID: 40872948 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

