Evaluation of the Immunological Efficacy of an LNP-mRNA Vaccine Prepared from Varicella Zoster Virus Glycoprotein gE with a Double-Mutated Carboxyl Terminus in Different Untranslated Regions in Mice
- PMID: 37766151
- PMCID: PMC10534744
- DOI: 10.3390/vaccines11091475
Evaluation of the Immunological Efficacy of an LNP-mRNA Vaccine Prepared from Varicella Zoster Virus Glycoprotein gE with a Double-Mutated Carboxyl Terminus in Different Untranslated Regions in Mice
Abstract
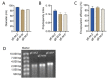
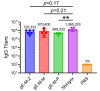
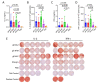
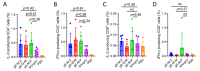
Cell-mediated immunity (CMI) plays a key role in the effectiveness of varicella zoster virus (VZV) vaccines, and mRNA vaccines have an innate advantage in inducing CMI. Glycoprotein E (gE) has been used widely as an antigen for VZV vaccines, and carboxyl-terminal mutations of gE are associated with VZV titer and infectivity. In addition, the untranslated regions (UTRs) of mRNA affect the stability and half-life of mRNA in the cell and are crucial for protein expression and antigenic translational efficiency. In this study, three UTRs were designed and connected to the nucleic acid sequence of gE-M, which is double mutated in the extracellular region of gE. Then, mRNA with different nucleic acids was encapsulated in lipid nanoparticles (LNPs), forming three LNP-mRNA VZV vaccines, named gE-M-Z, gE-M-M, and gE-M-P. The immune response elicited by these vaccines in mice was evaluated at intervals of 4 weeks, and the mice were sacrificed 2 weeks after the final immunization. In the results, the gE-M-P group, which retains the nucleic acid sequence of gE-M and is connected to Pfizer/BioNTech's BNT162b2 UTRs, induced the strongest humoral immune response and CMI. Because CMI is crucial for protection against VZV and for the design of VZV vaccines, this study provides a feasible strategy for improving the effectiveness and economy of future VZV vaccines.
Keywords: CMI; UTR; VZV; mRNA vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Arruti M., Pineiro L.D., Salicio Y., Cilla G., Goenaga M.A., Lopez de Munain A. Incidence of varicella zoster virus infections of the central nervous system in the elderly: A large tertiary hospital-based series (2007–2014) J. Neurovirol. 2017;23:451–459. doi: 10.1007/s13365-017-0519-y. - DOI - PubMed
-
- Asada H., Nagayama K., Okazaki A., Mori Y., Okuno Y., Takao Y., Miyazaki Y., Onishi F., Okeda M., Yano S., et al. An inverse correlation of VZV skin-test reaction, but not antibody, with severity of herpes zoster skin symptoms and zoster-associated pain. J. Dermatol. Sci. 2013;69:243–249. doi: 10.1016/j.jdermsci.2012.10.015. - DOI - PubMed
-
- Gilbert P.B., Gabriel E.E., Miao X., Li X., Su S.C., Parrino J., Chan I.S. Fold rise in antibody titers by measured by glycoprotein-based enzyme-linked immunosorbent assay is an excellent correlate of protection for a herpes zoster vaccine, demonstrated via the vaccine efficacy curve. J. Infect. Dis. 2014;210:1573–1581. doi: 10.1093/infdis/jiu279. - DOI - PMC - PubMed
Grants and funding
- 82104130/the National Natural Science Foundation of China
- 202301AT070362, 202201AU070150, and 202101AT070286/Yunnan Fundamental Research Projects
- 2022YFC2305700/the National Key R&D Program of China
- 202102AA310035/the Special Biomedicine Projects of Yunnan Province
- 202002AA100009/the Major Science and Technology Special Projects of Yunnan Province
- 3332022075/Fundamental Research Funds for the Central Universities
- H-2019063/Funds for the Training of High-Level Health Technical Personnel in Yunnan Province
- 202205AC160015/Funds for High-Level Scientific and Technological Talents Selection Special Project of Yunnan Province
- 2021-I2M-1-043/the CAMS Innovation Fund for Medical Sciences (CIFMS)
LinkOut - more resources
Full Text Sources
Research Materials

