Stimulation of Potent Humoral and Cellular Immunity via Synthetic Dual-Antigen MVA-Based COVID-19 Vaccine COH04S1 in Cancer Patients Post Hematopoietic Cell Transplantation and Cellular Therapy
- PMID: 37766168
- PMCID: PMC10538048
- DOI: 10.3390/vaccines11091492
Stimulation of Potent Humoral and Cellular Immunity via Synthetic Dual-Antigen MVA-Based COVID-19 Vaccine COH04S1 in Cancer Patients Post Hematopoietic Cell Transplantation and Cellular Therapy
Abstract
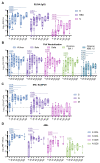
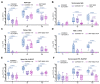
Hematopoietic cell transplantation (HCT) and chimeric antigen receptor (CAR)-T cell patients are immunocompromised, remain at high risk following SARS-CoV-2 infection, and are less likely than immunocompetent individuals to respond to vaccination. As part of the safety lead-in portion of a phase 2 clinical trial in patients post HCT/CAR-T for hematological malignancies (HM), we tested the immunogenicity of the synthetic modified vaccinia Ankara-based COVID-19 vaccine COH04S1 co-expressing spike (S) and nucleocapsid (N) antigens. Thirteen patients were vaccinated 3-12 months post HCT/CAR-T with two to four doses of COH04S1. SARS-CoV-2 antigen-specific humoral and cellular immune responses, including neutralizing antibodies to ancestral virus and variants of concern (VOC), were measured up to six months post vaccination and compared to immune responses in historical cohorts of naïve healthy volunteers (HV) vaccinated with COH04S1 and naïve healthcare workers (HCW) vaccinated with the FDA-approved mRNA vaccine Comirnaty® (Pfizer, New York, NY, USA). After one or two COH04S1 vaccine doses, HCT/CAR-T recipients showed a significant increase in S- and N-specific binding antibody titers and neutralizing antibodies with potent activity against SARS-CoV-2 ancestral virus and VOC, including the highly immune evasive Omicron XBB.1.5 variant. Furthermore, vaccination with COH04S1 resulted in a significant increase in S- and N-specific T cells, predominantly CD4+ T lymphocytes. Elevated S- and N-specific immune responses continued to persist at six months post vaccination. Furthermore, both humoral and cellular immune responses in COH04S1-vaccinated HCT/CAR-T patients were superior or comparable to those measured in COH04S1-vaccinated HV or Comirnaty®-vaccinated HCW. These results demonstrate robust stimulation of SARS-CoV-2 S- and N-specific immune responses including cross-reactive neutralizing antibodies by COH04S1 in HM patients post HCT/CAR-T, supporting further testing of COH04S1 in immunocompromised populations.
Keywords: COVID-19; SARS-CoV-2; cellular response; hematopoietic cell transplantation (HCT); humoral response; immunosuppression; modified vaccinia Ankara (MVA); nucleocapsid; phase 2 clinical trial; spike; vaccination.
Conflict of interest statement
While unknown whether publication of this report will aid in receiving grants and contracts, it is possible that this publication will be of benefit to City of Hope (COH). COH had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript. D.J.D. and F.W. are co-inventors on a patent application covering the design and construction of the synthetic MVA platform (PCT/US2021/016247). D.J.D., F.W., and F.C. are co-inventors on a patent application covering the development of a COVID-19 vaccine (PCT/US2021/032821). D.J.D. is a consultant for GeoVax. A.S. is a consultant for AstraZeneca Pharmaceuticals, Calyptus Pharmaceuticals, Inc, Darwin Health, EmerVax, EUROIMMUN, F. Hoffman-La Roche Ltd., Fortress Biotech, Gilead Sciences, Gritstone Oncology, Guggenheim Securities, Moderna, Pfizer, RiverVest Venture Partners, and Turnstone Biologics. LJI has filed for patent protection for various aspects of T cell epitope and vaccine design work. A.G. is a consultant for Pfizer. All other authors declare no competing interests. GeoVax Labs Inc. has taken a worldwide exclusive license for COH04S1 under the name of GEO-CM04S1.
Figures
Similar articles
-
Vaccine-induced spike- and nucleocapsid-specific cellular responses maintain potent cross-reactivity to SARS-CoV-2 Delta and Omicron variants.iScience. 2022 Aug 19;25(8):104745. doi: 10.1016/j.isci.2022.104745. Epub 2022 Jul 11. iScience. 2022. PMID: 35846380 Free PMC article.
-
Synthetic modified vaccinia Ankara vaccines confer cross-reactive and protective immunity against mpox virus.Commun Med (Lond). 2024 Feb 16;4(1):19. doi: 10.1038/s43856-024-00443-9. Commun Med (Lond). 2024. PMID: 38366141 Free PMC article.
-
Safety and immunogenicity of a synthetic multiantigen modified vaccinia virus Ankara-based COVID-19 vaccine (COH04S1): an open-label and randomised, phase 1 trial.Lancet Microbe. 2022 Apr;3(4):e252-e264. doi: 10.1016/S2666-5247(22)00027-1. Epub 2022 Mar 9. Lancet Microbe. 2022. PMID: 35287430 Free PMC article. Clinical Trial.
-
State of the CAR-T: Risk of Infections with Chimeric Antigen Receptor T-Cell Therapy and Determinants of SARS-CoV-2 Vaccine Responses.Transplant Cell Ther. 2021 Dec;27(12):973-987. doi: 10.1016/j.jtct.2021.09.016. Epub 2021 Sep 27. Transplant Cell Ther. 2021. PMID: 34587552 Free PMC article. Review.
-
The impact of COVID-19 on cancer patients.Cytokine Growth Factor Rev. 2024 Feb;75:110-118. doi: 10.1016/j.cytogfr.2023.11.004. Epub 2023 Nov 30. Cytokine Growth Factor Rev. 2024. PMID: 38103990 Review.
Cited by
-
Strategic and Technical Considerations in Manufacturing Viral Vector Vaccines for the Biomedical Advanced Research and Development Authority Threats.Vaccines (Basel). 2025 Jan 14;13(1):73. doi: 10.3390/vaccines13010073. Vaccines (Basel). 2025. PMID: 39852852 Free PMC article. Review.
-
New Trends in SARS-CoV-2 Variants and Vaccines.Vaccines (Basel). 2025 Mar 3;13(3):265. doi: 10.3390/vaccines13030265. Vaccines (Basel). 2025. PMID: 40266121 Free PMC article.
References
-
- Spanjaart A.M., Ljungman P., de La Camara R., Tridello G., Ortiz-Maldonado V., Urbano-Ispizua A., Barba P., Kwon M., Caballero D., Sesques P., et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: Results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia. 2021;35:3585–3588. doi: 10.1038/s41375-021-01466-0. - DOI - PMC - PubMed
-
- Varma A., Kosuri S., Ustun C., Ibrahim U., Moreira J., Bishop M.R., Nathan S., Mehta J., Moncayo D., Heng J., et al. COVID-19 infection in hematopoietic cell transplantation: Age, time from transplant and steroids matter. Leukemia. 2020;34:2809–2812. doi: 10.1038/s41375-020-01019-x. - DOI - PMC - PubMed
-
- Sharma A., Bhatt N.S., St Martin A., Abid M.B., Bloomquist J., Chemaly R.F., Dandoy C., Gauthier J., Gowda L., Perales M.A., et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study. Lancet Haematol. 2021;8:e185–e193. doi: 10.1016/S2352-3026(20)30429-4. - DOI - PMC - PubMed
-
- Maillard A., Redjoul R., Klemencie M., Labussiere Wallet H., Le Bourgeois A., D’Aveni M., Huynh A., Berceanu A., Marchand T., Chantepie S., et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood. 2022;139:134–137. doi: 10.1182/blood.2021014232. - DOI - PMC - PubMed
-
- Ni B., Yanis A., Dee K., Chappell J.D., Dulek D.E., Kassim A.A., Kitko C.L., Thomas L.D., Halasa N. SARS-CoV-2 vaccine safety and immunogenicity in patients with hematologic malignancies, transplantation, and cellular therapies. Blood Rev. 2022;56:100984. doi: 10.1016/j.blre.2022.100984. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

